Abstract
Background/Objective
Intraoperative evaluation of sentinel lymph nodes (SLNs) after neoadjuvant chemotherapy (NAC) has a higher false-negative rate than in the primary surgical setting, particularly for small tumor deposits. Additional tumor burden seen with isolated tumor cells (ITCs) and micrometastases following primary surgery is low; however, it is unknown whether the same is true after NAC. We examined the false-negative rate of intraoperative frozen section (FS) after NAC, and the association between SLN metastasis size and residual disease at axillary lymph node dissection (ALND).
Methods
Patients undergoing SLN biopsy after NAC were identified. The association between SLN metastasis size and residual axillary disease was examined.
Results
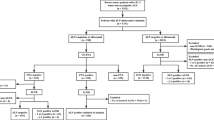
From July 2008 to July 2017, 702 patients (711 cancers) had SLN biopsy after NAC. On FS, 181 had metastases, 530 were negative; 33 negative cases were positive on final pathology (false-negative rate 6.2%). Among patients with a positive FS, 3 (2%) had ITCs and no further disease on ALND; 41 (23%) had micrometastases and 125 (69%) had macrometastases. Fifty-nine percent of patients with micrometastases and 63% with macrometastases had one or more additional positive nodes at ALND. Among those with a false-negative result, 10 (30%) had ITCs, 15 (46%) had micrometastases, and 8 (24%) had macrometastases; 17 had ALND and 59% had one or more additional positive lymph nodes. Overall, 1/6 (17%) patients with ITCs and 28/44 (64%) patients with micrometastases had additional nodal metastases at ALND.
Conclusion
Low-volume SLN disease after NAC is not an indicator of a low risk of additional positive axillary nodes and remains an indication for ALND, even when not detected on intraoperative FS.


Similar content being viewed by others
Change history
21 February 2020
The following are corrections to the original article.
References
Chu KU, Turner RR, Hansen NM, Brennan MB, Bilchik A, Giuliano AE. Do all patients with sentinel node metastasis from breast carcinoma need complete axillary node dissection? Ann Surg. 1999;229(4):536–41.
Turner RR, Chu KU, Qi K, Botnick LE, Hansen NM, Glass EC, Giuliano AE. Pathologic features associated with nonsentinel lymph node metastases in patients with metastatic breast carcinoma in a sentinel lymph node. Cancer. 2000;89(3):574–81.
Kamath VJ, Giuliano R, Dauway EL, Cantor A, Berman C, Ku NN, et al. Characteristics of the sentinel lymph node in breast cancer predict further involvement of higher-echelon nodes in the axilla: a study to evaluate the need for complete axillary lymph node dissection. Arch Surg. 2001;136(6):688–92.
Cserni G, Gregori D, Merletti F, Sapino A, Mano MP, Ponti A, et al. Meta-analysis of non-sentinel node metastases associated with micrometastatic sentinel nodes in breast cancer. Br J Surg. 2004;91(10):1245–52.
Viale G, Maiorano E, Pruneri G, Mastropasqua MG, Valentini S, Galimberti V, et al. Predicting the risk for additional axillary metastases in patients with breast carcinoma and positive sentinel lymph node biopsy. Ann Surg. 2005;241(2):319–25.
van Deurzen CH, de Boer M, Monninkhof EM, Bult P, van der Wall E, Tjan-Heijnen VC, et al. Non-sentinel lymph node metastases associated with isolated breast cancer cells in the sentinel node. J Natl Cancer Inst. 2008;100(22):1574–80.
Houvenaeghel G, Nos C, Giard S, Mignotte H, Esterni B, Jacquemier J, et al. A nomogram predictive of non-sentinel lymph node involvement in breast cancer patients with a sentinel lymph node micrometastasis. Eur J Surg Oncol. 2009;35(7):690–5.
Van Zee KJ, Manasseh DM, Bevilacqua JL, Boolbol SK, Fey JV, Tan LK, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10(10):1140–1.
Kumar S, Bramlage M, Jacks LM, Goldberg JI, Patil SM, Giri DD, et al. Minimal disease in the sentinel lymph node: how to best measure sentinel node micrometastases to predict risk of additional non-sentinel lymph node disease. Ann Surg Oncol. 2010;17(11):2909–9.
Akay CL, Albarracin C, Torstenson T, Bassett R, Mittendorf EA, Yi M, et al. Factors impacting the accuracy of intra-operative evaluation of sentinel lymph nodes in breast cancer. Breast J. 2018;24(1):28–34.
Rubio IT, Aznar F, Lirola J, Peg V, Xercavins J. Intraoperative assessment of sentinel lymph nodes after neoadjuvant chemotherapy in patients with breast cancer. Ann Surg Oncol. 2010;17(1):235–9.
Komenaka IK, Torabi R, Nair G, Jayaram L, Hsu CH, Bouton ME, et al. Intraoperative touch imprint and frozen section analysis of sentinel lymph nodes after neoadjuvant chemotherapy for breast cancer. Ann Surg. 2010;251(2):319–22.
Shimazu K, Tamaki Y, Taguchi T, Tsukamoto F, Kasugai T, Noguchi S. Intraoperative frozen section analysis of sentinel lymph node in breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2008;15(6):1717–22.
Weiser MR, Montgomery LL, Susnik B, Tan LK, Borgen PI, Cody HS. Is routine intraoperative frozen-section examination of sentinel lymph nodes in breast cancer worthwhile? Ann Surg Oncol. 2000;7(9):651–5.
American Joint Commission on Cancer. AJCC cancer staging manual breast cancer, 7th edition. https://cancerstaging.org/references-tools/quickreferences/Documents/BreastMedium.pdf. Accessed Nov 2018.
Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14(4):297–305.
Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (alliance) randomized clinical trial. JAMA. 2017;318(10):918–26.
National Comprehensive Cancer Network. Clinical Practice Guidelines Oncology—Breast Cancer (Version 2.2017). 2017. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 24 Jul 2017.
Gimbergues P, Dauplat MM, Durando X, Abrial C, Le Bouedec G, Mouret-Reynier MA, et al. Intraoperative imprint cytology examination of sentinel lymph nodes after neoadjuvant chemotherapy in breast cancer patients. Ann Surg Oncol. 2010;17(8):2132–7.
Espinosa-Bravo M, Navarro-Cecilia J, Boyero MR, Diaz-Botero S, Rodriguez BD, Lopez CL, et al. Intraoperative assessment of sentinel lymph node by one-step nucleic acid amplification in breast cancer patients after neoadjuvant treatment reduces the need for a second surgery for axillary lymph node dissection. Breast. 2017;31:40–5.
Donker M, Slaets L, van Tienhoven G, Rutgers EJ. Axillary lymph node dissection versus axillary radiotherapy in patients with a positive sentinel node: the AMAROS trial [in Dutch]. Ned Tijdschr Geneeskd. 2015;159:A9302.
Galimberti V, Ribeiro Fontana SK, Maisonneuve P, Steccanella F, Vento AR, Intra M, Naninato P, et al. Sentinel node biopsy after neoadjuvant treatment in breast cancer: Five-year follow-up of patients with clinically node-negative or node-positive disease before treatment. Eur J Surg Oncol. 2016;42(3):361–8.
Jeruss JS, Newman LA, Ayers GD, Cristofanilli M, Broglio KR, Meric-Bernstam F, et al. Factors predicting additional disease in the axilla in patients with positive sentinel lymph nodes after neoadjuvant chemotherapy. Cancer. 2008;112(12):2646–54.
Zetterlund LH, Frisell J, Zouzos A, Axelsson R, Hatschek T, de Boniface J, et al. Swedish prospective multicenter trial evaluating sentinel lymph node biopsy after neoadjuvant systemic therapy in clinically node-positive breast cancer. Breast Cancer Res Treat. 2017;163(1):103–10.
Kantor O, Pesce C, Liederbach E, Wang CH, Winchester DJ, Yao K. Are the ACOSOG Z0011 trial findings being applied to breast cancer patients undergoing neoadjuvant chemotherapy? Breast J. 2017;23(5):554–62.
Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, et al. Axillary dissection versus no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–75.
Disclosures
Tracy-Ann Moo, Marcia Edelweiss, Sabina Hajiyeva, Michelle Stempel, Monica Raiss, Emily C. Zabor, Andrea Barrio, and Monica Morrow have no conflicts of interest disclosures to report.
Funding
The preparation of this study was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moo, TA., Edelweiss, M., Hajiyeva, S. et al. Is Low-Volume Disease in the Sentinel Node After Neoadjuvant Chemotherapy an Indication for Axillary Dissection?. Ann Surg Oncol 25, 1488–1494 (2018). https://doi.org/10.1245/s10434-018-6429-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6429-2