Abstract
Background
Cost-effectiveness evaluations of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment of peritoneal carcinomatosis (PC) from metastatic colorectal cancer (mCRC) in the United States are lacking.
Methods
The authors developed a Markov model to evaluate the cost-effectiveness of CRS/HIPEC compared with systemic chemotherapy for isolated PC from mCRC from a societal perspective in the United States. The systemic treatment regimens consisted of FOLFOX, FOLFIRI, bevacizumab, cetuximab, and pantitumumab. The model inputs including costs, probabilities, survival, progression, and utilities were taken from the literature. The cycle length for the model was 2 weeks, and the time horizon was 7 years. A discount rate of 3% was applied. The model was tested for internal and external validation, and robustness was established with univariate sensitivity and probabilistic sensitivity analyses (PSA). The primary outcomes were total costs, quality-adjusted life-years (QALYs), life-years (LYs), and incremental cost-effectiveness ratio (ICER). A willingness-to-pay (WTP) threshold of $100,000 per QALY was assumed.
Results
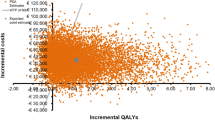
The ICER for treatment with CRS/HIPEC compared with systemic chemotherapy was $91,034 per QALY gained ($74,098 per LY gained). The univariate sensitivity analysis showed that the total costs for treatment with CRS/HIPEC had the largest effect on the calculated ICER. The CRS/HIPEC treatment was a cost-effective strategy during the majority of simulations in the PSA. The average ICER for 100,000 simulations in the PSA was $70,807 per QALY gained. The likelihood of CRS/HIPEC being a cost-effective strategy at the WTP threshold was 87%.
Conclusions
The CRS/HIPEC procedure is a cost-effective treatment for isolated PC from mCRC in the United States.



Similar content being viewed by others
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. https://doi.org/10.3322/caac.21208.
Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545–50. https://doi.org/10.1046/j.1365-2168.2002.02274.x.
Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of North Central Cancer Treatment Group phase III trials N9741 and N9841. J Clin Oncol. 2012;30:263–7. https://doi.org/10.1200/jco.2011.37.1039.
Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535–46. https://doi.org/10.1093/annonc/mdq632.
Van Cutsem E, Kohne C-H, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17.
Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–43. https://doi.org/10.1200/jco.2003.04.187.
Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ III. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116:3756–62. https://doi.org/10.1002/cncr.25116.
Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27:681–5. https://doi.org/10.1200/jco.2008.19.7160.
Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284–92. https://doi.org/10.1200/jco.2004.10.012.
Bijelic L, Yan TD, Sugarbaker PH. Treatment failure following complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from colorectal or appendiceal mucinous neoplasms. J Surg Oncol. 2008;98:295–9. https://doi.org/10.1002/jso.21084.
Turaga K, Levine E, Barone R, et al. Consensus guidelines from the American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann Surg Oncol. 2013;21:1501–5. https://doi.org/10.1245/s10434-013-3061-z.
Chua TC, Esquivel J, Pelz JOW, Morris DL. Summary of current therapeutic options for peritoneal metastases from colorectal cancer. J Surg Oncol. 2013;107:566–73. https://doi.org/10.1002/jso.23189.
Esquivel J, Piso P, Verwaal V, et al. American Society of Peritoneal Surface Malignancies opinion statement on defining expectations from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with colorectal cancer. J Surg Oncol. 2014;110:777–8. https://doi.org/10.1002/jso.23722.
Bonastre J, Chevalier J, Elias D, et al. Cost-effectiveness of intraperitoneal chemohyperthermia in the treatment of peritoneal carcinomatosis from colorectal cancer. Value Health. 2008;11:347–53. https://doi.org/10.1111/j.1524-4733.2007.00249.x.
Chua TC, Martin S, Saxena A, et al. Evaluation of the cost-effectiveness of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (peritonectomy) at the St George Hospital Peritoneal Surface Malignancy Program. Ann Surg. 2010;251:323–9. https://doi.org/10.1097/sla.0b013e3181c9b53c.
Vanounou T, Garfinkle R. Evaluation of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin in the era of value-based medicine. Ann Surg Oncol. 2016;23:2556–61. https://doi.org/10.1245/s10434-016-5096-4.
Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9. https://doi.org/10.1200/jco.2007.14.9930.
Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. https://doi.org/10.1200/jco.2004.05.113.
Karapetis C, Khambata-Ford S, Jonker D, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65.
Centers for Medicare and Medicaid Services. 2017. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-F.html. Accessed 25 Feb 2018.
Centers for Disease Control and Prevention. 2017. https://www.cdc.gov/nchs/fastats/body-measurementshtm. Accessed 25 Feb 2018.
Centers for Medicare and Medicaid Services. 2017. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/. Accessed 25 Feb 2018.
Dindo D, Demartines N, Clavien P-A. Classification of surgical complications. Ann Surg. 2004;240:205–13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
Lyman GH, Bohlke K, Falanga A. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. JOP. 2015;11:e442–4. https://doi.org/10.1200/jop.2015.004473.
Dodson RM, McQuellon RP, Mogal HD, et al. Quality-of-life evaluation after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2016;23:772–83. https://doi.org/10.1245/s10434-016-5547-y.
Duriseti RS, Brandeau ML. Cost-effectiveness of strategies for diagnosing pulmonary embolism among emergency department patients presenting with undifferentiated symptoms. Ann Emerg Med. 2010;56:321–32. https://doi.org/10.1016/j.annemergmed.2010.03.029.
Ara R, Brazier J. Deriving an algorithm to convert the eight mean SF-36 dimension scores into a mean EQ-5D preference-based score from published studies (where patient-level data are not available). Value Health. 2008;11:1131–43. https://doi.org/10.1111/j.1524-4733.2008.00352.x.
Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-Year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–32. https://doi.org/10.1245/s10434-008-9966-2.
Pietro F Bagnoli, Cananzi FCM, Brocchi A, et al. Peritonectomy and hyperthermic intraperitoneal chemotherapy: cost analysis and sustainability. Eur J Surg Oncol. 2015;41:386–91. https://doi.org/10.1016/j.ejso.2014.12.004.
Baratti D, Scivales A, Balestra MR, et al. Cost analysis of the combined procedure of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Surg Oncol. 2010;36:463–9. https://doi.org/10.1016/j.ejso.2010.03.005.
Naffouje SA, O’Donoghue C, Salti GI. Evaluation of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a community setting: a cost-utility analysis of a hospital’s initial experience and reflections on the health care system. J Surg Oncol. 2016;113:544–7. https://doi.org/10.1002/jso.24162.
Squires MH, Staley CA, Knechtle W, et al. Association between hospital finances, payer mix, and complications after hyperthermic intraperitoneal chemotherapy: deficiencies in the current healthcare reimbursement system and future implications. Ann Surg Oncol. 2015;22:1739–45. https://doi.org/10.1245/s10434-014-4025-7.
Tentes AA, Pallas N, Korakianitis O, et al. The cost of cytoreductive surgery and perioperative intraperitoneal chemotherapy in the treatment of peritoneal malignancy in one Greek institute. J BUON. 2012;17:776–80.
Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–81.
NCCN Guidelines–Colon Cancer. Plymouth Meeting, PA: Harborside Press; 2017. pp. 1–143.
Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–91. https://doi.org/10.1016/s1470-2045(17)30422-9.
Klaver YLB, Chua TC, Verwaal VJ, de Hingh IHJT, Morris DL. Secondary cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal recurrence of colorectal and appendiceal peritoneal carcinomatosis following prior primary cytoreduction. J Surg Oncol. 2013;107:585–90. https://doi.org/10.1002/jso.23303.
Hamilton TD, Taylor EL, Cannell AJ, McCart JA. Impact of major complications on patients’ quality of life after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2016;23:2946–52. https://doi.org/10.1245/s10434-016-5231-2.
Weinstein M, Skinner J. Comparitive effectiveness and health care spending: implications for reform. N Engl J Med. 2010;362:460–5.
Braithwaite RS, Meltzer DO, King JT, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–56.
Quenet F, Elias D, Roca L, et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J Clin Oncol. 2018;36(18 Suppl):3503. https://doi.org/10.1200/jco.2018.36.18_suppl.lba3503.
Esquivel J, Lowy AM, Markman M, et al. The American Society of Peritoneal Surface Malignancies (ASPSM) multiinstitution evaluation of the Peritoneal Surface Disease Severity Score (PSDSS) in 1013 Patients with colorectal cancer with peritoneal carcinomatosis. Ann Surg Oncol. 2014;21:4195–201. https://doi.org/10.1245/s10434-014-3798-z.
Jafari MD, Halabi WJ, Stamos MJ, et al. Surgical outcomes of hyperthermic intraperitoneal chemotherapy. JAMA Surg. 2014;149:170–6. https://doi.org/10.1001/jamasurg.2013.3640.
Shen P, Thai K, Stewart JH, et al. Peritoneal surface disease from colorectal cancer: comparison with the hepatic metastases surgical paradigm in optimally resected patients. Ann Surg Oncol. 2008;15:3422–32. https://doi.org/10.1245/s10434-008-0127-4.
Kianmanesh R, Scaringi S, Sabate J-M, et al. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg. 2007;245:597–603. https://doi.org/10.1097/01.sla.0000255561.87771.11.
Hinkle NM, MacDonald J, Sharpe JP, Dickson P, Deneve J, Munene G. Cytoreduction with hyperthermic intraperitoneal chemotherapy: an appraisal of outcomes and cost at a newly established peritoneal malignancy program. Am J Surg. 2016;212:413–8. https://doi.org/10.1016/j.amjsurg.2016.01.022.
Amado RG, Wolf M, Peeters M, et al. Wild-type KRASIs required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. https://doi.org/10.1200/jco.2007.14.7116.
McQuellon RP, Danhauer SC, Russell GB, et al. Monitoring health outcomes following cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2007;14:1105–13. https://doi.org/10.1245/s10434-006-9304-5.
Bennett L, Zhao Z, Barber B, et al. Health-related quality of life in patients with metastatic colorectal cancer treated with panitumumab in first- or second-line treatment. Br J Cancer. 2011;105:1495–502. https://doi.org/10.1038/bjc.2011.409.
Odom D, Barber B, Bennett L, et al. Health-related quality of life and colorectal cancer-specific symptoms in patients with chemotherapy-refractory metastatic disease treated with panitumumab. Int J Colorectal Dis. 2011;26:173–81. https://doi.org/10.1007/s00384-010-1112-5.
Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. The Lancet. 2013;381:303–12. https://doi.org/10.1016/s0140-6736(12)61900-x.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

10434_2018_7111_MOESM1_ESM.jpg
Fig A1. Markov model output for overall survival from CRS/HIPEC versus systemic chemotherapy (A); published survival results from randomized trial comparing CRS/HIPEC versus systemic chemotherapy28 (B); published survival results from large cohort treated with CRS/HIPEC and systemic chemotherapy7 (C). (JPEG 381 kb)


Rights and permissions
About this article
Cite this article
Hamilton, T.D., MacNeill, A.J., Lim, H. et al. Cost-Effectiveness Analysis of Cytoreductive Surgery and HIPEC Compared With Systemic Chemotherapy in Isolated Peritoneal Carcinomatosis From Metastatic Colorectal Cancer. Ann Surg Oncol 26, 1110–1117 (2019). https://doi.org/10.1245/s10434-018-07111-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-07111-y