Abstract
Background
Counterintuitively, more deaths from melanoma occur among patients with thin (T1) primary melanomas (≤ 1 mm) than among those with thick primary melanoma because the great majority present with T1 tumors. Therefore, it is important to stratify their risk as accurately as possible to guide their management and follow-up. This study sought to explore the relationship between tumor thickness and prognosis for patients with thin primary melanomas.
Methods
A retrospective, single-institution study investigated 6263 patients with cutaneous melanoma (including 2117 T1 cases) who had a minimum follow-up period of 10 years.
Results
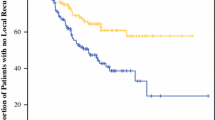
For the entire patient cohort, the 10-year melanoma-specific survival (MSS) rate ranged between 92% for the patients with primary melanomas up to 0.3 mm thick and 32% for those with melanomas thicker than 8 mm. When divided into 25-quantile-thickness groups there was a significant difference in 10-year MSS between the two consecutive groups 0.8 and 0.9 mm; the differences in survival were not significantly different for any other consecutive cut points within the less than or equal to 1 mm thickness range, indicating a biologically-relevant difference in outcome above and below 0.8 mm. For the patients treated initially at the authors’ institution, the 10- and 20-year MSS rates for those with tumors up to 0.8 mm thick were respectively 93.4 and 85.7%, and for tumors 0.9 to 1.0 mm, the rates were respectively 81.1 and 71.4%. Only 29.3% of the T1 patients who died of melanoma were deceased within 5 years.
Conclusions
A naturally occurring thickness cut point of 0.8 mm predicts higher or lower risk for patients with thin primary cutaneous melanomas. Long-term follow-up assessment of patients with T1 melanoma is important because late mortality due to melanoma is more common than early mortality.


Similar content being viewed by others
References
Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206.
Balch CM, Soong SJ, Shaw HM, Milton GW. An analysis of prognostic factors in 8500 patients with cutaneous melanoma. In: Balch CM, Houghton AN, Milton GW, Sober AJ, Soong SJ, editors. Cutaneous melanoma: clinical management and treatment results worldwide. 2nd ed. JB Lipincott, Philadelphia, 1992, pp. 165–187.
Breslow A. Thickness, cross-sectional areas, and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902–8.
Garbe C, Buttner P, Bertz J, Burg G, d’Hoedt B, Drepper H, et al. Primary cutaneous melanoma: identification of prognostic groups and estimation of individual prognosis for 5093 patients. Cancer. 1995;75:2484–91.
Masback A, Olsson H, Westerdahl J, Ingvar C, Jonsson N. Prognostic factors in invasive cutaneous malignant melanoma: a population-based study and review. Melanoma Res. 2001;11:435–45.
Scolyer RA, Judge MJ, Evans A, Frishberg DP, Prieto VG, Thompson JF, et al, International collaboration on cancer R. Data set for pathology reporting of cutaneous invasive melanoma: recommendations from the international collaboration on cancer reporting (ICCR). Am J Surg Pathol. 2013;37:1797–814.
Buettner P, Garbe C, Guggenmoos-Holzmann I. Problems in defining cutoff points of continuous prognostic factors: example of tumor thickness in primary cutaneous melanoma. J Clin Epidemiol. 1997;50:1201–10.
Gimotty PA, Elder DE, Fraker DL, Botbyl J, Sellers K, Elenitsas R, et al. Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J Clin Oncol. 2007;25:1129–34.
Kalady MF, White RR, Johnson JL, Tyler DS, Seigler HF. Thin melanomas: predictive lethal characteristics from a 30-year clinical experience. Ann Surg. 2003;238:528–35.
Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cut points in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86:829–35.
Contal C, O’Quigley J. An application of change-point methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30:253–70.
Faraggi D, Simon R. A simulation study of cross-validation for selecting an optimal cut point in univariate survival analysis. Stat Med. 1996;15:2203–13.
Hilsenbeck SG, Clark GM. Practical p value adjustment for optimally selected cut points. Stat Med. 1996;15:103–12.
Hilsenbeck SG, Clark GM, McGuire WL. Why do so many prognostic factors fail to pan out? Breast Cancer Res Treat. 1992;22:197–206.
Amin MB, Edge SB, Greene FL, Carducci MA, Compton CA, editors. AJCC Cancer Staging Manual. 8th ed. New York: Springer International Publishing: 2017.
Cumming G, Finch S. Inference by eye: confidence intervals and how to read pictures of data. Am Psychol. 2005;60:170–80.
Woodward M. Epidemiology: study design and data analysis. 2nd ed. Boca Raton: Chapman and Hall/CRC Press; 2005.
Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin. 2017;67:472–92.
Green AC, Baade P, Coory M, Aitken JF, Smithers M. Population-based 20-year survival among people diagnosed with thin melanomas in Queensland, Australia. J Clin Oncol. 2012;30:1462–7.
Mansson-Brahme E, Carstensen J, Erhardt K, Lagerlof B, Ringborg U, Rutqvist LE. Prognostic factors in thin cutaneous malignant melanoma. Cancer. 1994;73:2324–32.
Balch CM, Wilkerson JA, Murad TM, Soong SJ, Ingalls AL, Maddox WA. The prognostic significance of ulceration of cutaneous melanoma. Cancer. 1980;45:3012–7.
McKinnon JG, Yu XQ, McCarthy WH, Thompson JF. Prognosis for patients with thin cutaneous melanoma: long-term survival data from New South Wales Central Cancer Registry and the Sydney Melanoma Unit. Cancer. 2003;98:1223–31.
Shaw HM, McCarthy WH, McCarthy SW, Milton GW. Thin malignant melanomas and recurrence potential. Arch Surg. 1987;122:1147–50.
Yonick DV, Ballo RM, Kahn E, Dahiya M, Yao K, Godellas C, et al. Predictors of positive sentinel lymph node in thin melanoma. Am J Surg. 2011;201:324–7.
Keefe M, Mackie RM. The relationship between risk of death from clinical stage 1 cutaneous melanoma and thickness of primary tumour: no evidence for steps in risk. Br J Cancer. 1991;64:598–602.
Clark WH Jr, From L, Bernardino EA, et al. The histogenesis and biological behavior of primary human malignant melanoma of the skin. Cancer Res. 1969;29:705–27.
Scolyer R, Shaw H, Thompson J, Li L, Colman M, Lo S, et al. Interobserver reproducibility of histopathologic prognostic variables in primary cutaneous melanomas. Am J Surg Pathol. 2003;27:1571–6.
Murali R, Hughes M, FitzGerald P, Thompson J, Scolyer R. Interobserver variation in the histopathologic reporting of key prognostic parameters, particularly Clark level, affects pathologic staging of primary cutaneous melanoma. Ann Surg. 2009;249:641–7.
Braun RP, Gutkowicz-Krusin D, Rabinovitz H, Cognetta A, Hofmann-Wellenhof R, Ahlgrimm-Siess V, et al. Agreement of dermatopathologists in the evaluation of clinically difficult melanocytic lesions: how golden is the “gold standard”? Dermatology. 2012;224:51–8.
Glusac EJ. The melanoma “epidemic”: lessons from prostate cancer. J Cutan Pathol. 2012;39:17–20.
Mihic-Probst D, Shea C, Duncan L, de la Fouchardiere A, Landman G, Landsberg J, et al. Update on thin melanoma: outcome of an international workshop. Adv Anat Pathol. 2016;23:24–9.
Monheit G, Cognetta AB, Ferris L, Rabinovitz H, Gross K, Martini M, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188–94.
Disclosure
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lo, S.N., Scolyer, R.A. & Thompson, J.F. Long-Term Survival of Patients with Thin (T1) Cutaneous Melanomas: A Breslow Thickness Cut Point of 0.8 mm Separates Higher-Risk and Lower-Risk Tumors. Ann Surg Oncol 25, 894–902 (2018). https://doi.org/10.1245/s10434-017-6325-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-6325-1