Abstract
Background
Residual disease (RD) after primary debulking surgery (PDS) is one of the main factors driving ovarian cancer prognosis. The primary end point of this study was assessment of the impact that surgery had on survival outcomes for patients with advanced ovarian cancer.
Methods
Data on the effect of newly diagnosed advanced-stage ovarian, tubal, and peritoneal cancers were analyzed during two study periods (T1: 2001–2006 and T2: 2007–2012), in which the concepts of optimal and complete cytoreduction were introduced and implemented.
Results
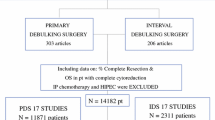
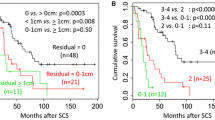
In this study, 260 patients (36%) had surgery during T1 and 462 patients (64%) had surgery during T2. The rate of PDS increased, from 55.4% (144/260) during T1 to 85.5% (395/462) during T2 (p < 0.001). At the time of PDS, complete resection (RD0) was achieved for 45.1% of the patients during T1 and 76.7% of the patients during T2 (p < 0.001), whereas optimal resection (RD < 1 cm) was achieved for 60.4% of the patients during T1 and 85.3% of the patients during T2 (p < 0.001). Disease-free survival improved during the study periods (p = 0.006). Overall survival was similar in T1 and T2 (p = 0.18). The preoperative CA125 level, disease stage, and RD remained independently associated with disease-free survival (p ≤ 0.05). The performance of interval debulking surgery (IDS) instead of PDS correlated with worse survival outcomes (hazard ratio [HR] 1.47; 95% confidence interval [CI] 1.24–1.92; p = 0.02), whereas achievement of RD0 and RD < 1 cm independently improved overall survival (HR 0.45; 95% CI 0.22–0.91; p = 0.02 for RD0 and HR 0.47; 95% CI 0.23–0.96; p = 0.03 for RD0).
Conclusions
The implementation of extensive cytoreduction allows improvement of patient outcomes. Further studies are needed to assess the risk-to-benefit ratio between PDS and IDS and to identify patients who benefit much more from one treatment method than from another.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Sioulas VD, Schiavone MB, Kadouri D, et al. Optimal primary management of bulky stage IIIC ovarian, fallopian tube and peritoneal carcinoma: are the only options complete gross resection at primary debulking surgery or neoadjuvant chemotherapy? Gynecol Oncol. 2017;145:15–20.
Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170:974–9.
Chang SJ, Hodeib M, Chang J, Bristow RE. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol. 2013;130:493–8.
Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–59.
Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–53.
Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–57.
Onda T, Satoh T, Saito T, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV. Ovarian/tubal/peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer. 2016;64:22–31.
Fagotti A, Ferrandina G, Vizzielli G, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): final analysis of peri-operative outcome. Eur J Cancer. 2016;59:22–33.
Mahner S, Trillsch F, Chi D, et al. Neoadjuvant chemotherapy in ovarian cancer revisited. Ann Oncol. 2016;1:i30–i32.
Hutter MM, Rowell KS, Devaney LA, Sokal SM, Warshaw AL, Abbott WM, Hondin RA. Identification of surgical complications and deaths: an assessment of the traditional surgical morbidity and mortality conference compared with the American College of Surgeons-National Surgical Quality Improvement Program. J Am Coll Surg. 2006;203:618–24.
Ditto A, Martinelli F, Bogani G, et al. Long-term safety of fertility-sparing surgery in early-stage ovarian cancer: comparison to standard radical surgical procedures. Gynecol Oncol. 2015;138:78–82.
Raspagliesi F, Ditto A, Martinelli F, Haeusler E, Lorusso D. Advanced ovarian cancer: omental bursa, lesser omentum, celiac, portal and triad nodes spread as cause of inaccurate evaluation of residual tumor. Gynecol Oncol. 2013;129:92–6.
Eastern Cooperative Oncology Group. ECOG performance status. http://ecog-acrin.org/resources/ecog-performance-status. Accessed 2 July 2016.
Bogani G, Uccella S, Ghezzi F. Impact of comorbidity index on survival in endometrial cancer. Am J Clin Oncol. 2014;37:642–3.
Wagner U. Lymphadenectomy in Ovarian Neoplasms (LION) protocol (AGO-OVAR OP.3). www.clinicaltrials.gov. Accessed 2 July 2016.
Ataseven B, Chiva LM, Harter P, Gonzalez-Martin A, du Bois A. FIGO stage IV epithelial ovarian, fallopian tube, and peritoneal cancer revisited. Gynecol Oncol. 2016. doi:10.1016/j.ygyno.2016.06.013.
Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol. 2007;197:676.e1–7.
Bogani G, Multinu F, Dowdy SC, et al. Incorporating robotic-assisted surgery for endometrial cancer staging: analysis of morbidity and costs. Gynecol Oncol. 2016;141:218–24.
Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. 1975;42:101–4.
du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009;115:1234–44.
Chi DS, Eisenhauer EL, Zivanovic O, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114:26–31.
Chi DS, Palayekar MJ, Sonoda Y, et al. Nomogram for survival after primary surgery for bulky stage IIIC ovarian carcinoma. Gynecol Oncol. 2008;108:191–4.
Aletti GD, Dowdy SC, Gostout BS, et al. Quality improvement in the surgical approach to advanced ovarian cancer: the Mayo Clinic experience. J Am Coll Surg. 2009;208:614–20.
Peiretti M, Zanagnolo V, Aletti GD, et al. Role of maximal primary cytoreductive surgery in patients with advanced epithelial ovarian and tubal cancer: surgical and oncological outcomes: single-institution experience. Gynecol Oncol. 2010;119:259–64.
Chiva LM, Castellanos T, Alonso S, Gonzalez-Martin A. Minimal macroscopic residual disease (0.1–1 cm): is it still a surgical goal in advanced ovarian cancer? Int J Gynecol Cancer. 2016;26:906–11.
Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:3460–73.
Aletti GD, Garbi A, Messori P, et al. Multidisciplinary approach in the management of advanced ovarian cancer patients: a personalized approach: results from a specialized ovarian cancer unit. Gynecol Oncol. 2017;144:468–73.
Aletti GD, Santillan A, Eisenhauer EL, et al. A new frontier for quality of care in gynecologic oncology surgery: multi-institutional assessment of short-term outcomes for ovarian cancer using a risk-adjusted model. Gynecol Oncol. 2007;107:99–106.
Petrillo M, Paris I, Vizzielli G, et al. Neoadjuvant chemotherapy followed by maintenance therapy with or without bevacizumab in unresectable high-grade serous ovarian cancer: a case-control study. Ann Surg Oncol. 2015;22:S952–8.
Kessous R, Laskov I, Abitbol J, et al. Clinical outcome of neoadjuvant chemotherapy for advanced ovarian cancer. Gynecol Oncol. 2017;144:474–9.
Karam A, Ledermann JA, Kim JW, et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: first-line interventions. Ann Oncol. 2017;28:711–7.
Di Donato V, Kontopantelis E, Aletti G, et al. Trends in mortality after primary cytoreductive surgery for ovarian cancer: a systematic review and metaregression of randomized clinical trials and observational studies. Ann Surg Oncol. 2017;24:1688–97.
Disclosure
Francesco Raspagliesi declared previous personal fees for board from Roche and PharmaMar. Giorgio Bogani declared previous personal fees for board from Novartis AG Pharma. Domenica Lorusso declared previous grants and personal fees for board from PharmaMar and previous personal fees for board from Roche and AstraZeneca. The remaining authors reported no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raspagliesi, F., Bogani, G., Ditto, A. et al. Implementation of Extensive Cytoreduction Resulted in Improved Survival Outcomes for Patients with Newly Diagnosed Advanced-Stage Ovarian, Tubal, and Peritoneal Cancers. Ann Surg Oncol 24, 3396–3405 (2017). https://doi.org/10.1245/s10434-017-6030-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-6030-0