Abstract
Background
A prospective clinical trial of combination neoadjuvant chemotherapy, transoral robotic surgery (TORS), and customized adjuvant therapy for patients with locally advanced oropharyngeal cancer was conducted.
Methods
Between July 2009 and October 2016, 31 patients were enrolled in this clinical trial.
Results
The primary lesions were located in the tonsils of 27 patients and in the base of the tongue of 4 patients. Of the 31 patients, 16 (51.6%) were classified as T3 and 15 patients (48.4%) as T4a. Three patients (9.7%) had stage 3 disease, and 28 (90.3%) had stage 4 disease. The 5-year overall survival rate was 78.7%; the 5-year disease-specific survival rate was 85%; and the 5-year disease-free survival rate was 80.8%. At the final follow-up visit, 26 patients were alive with no evidence of disease, and 1 was alive with disease. Four patients died during the study: two of tumor-node-metastasis (TNM)-related disease and two of another condition. All the patients tolerated an oral diet at an average of 7.4 days postoperatively. At the subjective swallowing evaluation using the Functional Outcome Swallowing Scale score, 83.9% of the patients exhibited favorable outcomes. No patient was permanently dependent on a feeding tube. All the patients breathed and phonated in the absence of a permanent tracheotomy at the final follow-up evaluation.
Conclusions
The treatment strategy in this study afforded good oncologic and functional outcomes for patients with locally advanced oropharyngeal cancer. Although future large-scale multicenter studies with longer follow-up periods are needed, this study showed that neoadjuvant chemotherapy combined with TORS is useful for treating advanced oropharyngeal cancer.


Similar content being viewed by others
References
Dörr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol. 2001;61:223–31.
Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–39.
Fung K, Lyden TH, Lee J, et al. Voice and swallowing outcomes of an organ-preservation trial for advanced laryngeal cancer. Int J Radiat Oncol Biol Phys. 2005;63:1395–9.
Rieger JM, Zalmanowitz JG, Wolfaardt JF. Functional outcomes after organ preservation treatment in head and neck cancer: a critical review of the literature. Int J Oral Maxillofac Surg. 2006;35:581–7.
Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–9.
Spiro RH, Gerold FP, Strong EW. Mandibular “swing” approach for oral and oropharyngeal tumors. Head Neck Surg. 1981;3:371–8.
Barrs DM, DeSanto LW, O’Fallon WM. Squamous cell carcinoma of the tonsil and tongue-base region. Arch Otolaryngol. 1979;105:479–85.
Cohen EE, LaMonte SJ, Erb NL, et al. American Cancer Society Head and Neck Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66:203–39.
Goldfarb M, Gondek S, Hodin R, Parangi S. Resident/fellow assistance in the operating room for endocrine surgery in the era of fellowships. Surgery. 2010;148:1065–71.
Weinstein GS, O’Malley BW Jr, Magnuson JS, et al. Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope. 2012;122:1701–7.
Weinstein GS, Quon H, Newman HJ, et al. Transoral robotic surgery alone for oropharyngeal cancer: an analysis of local control. Arch Otolaryngol Head Neck Surg. 2012;138:628–34.
Weinstein GS, O’Malley BW Jr, Cohen MA, Quon H. Transoral robotic surgery for advanced oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2010;136:1079–85.
Couch ME, Zanation A. Transoral robotic surgery: disruptive or sustaining innovation? Arch Surg. 2010;145:907–8.
Park YM, Lee JG, Lee WS, Choi EC, Chung SM, Kim SH. Feasibility of transoral lateral oropharyngectomy using a robotic surgical system for tonsillar cancer. Oral Oncol. 2009;45:e62–6.
Park YM, Kim WS, Byeon HK, Lee SY, Kim SH. Oncological and functional outcomes of transoral robotic surgery for oropharyngeal cancer. Br J Oral Maxillofac Surg. 2013;51:408–12.
Salassa JR. A functional outcome swallowing scale for staging oropharyngeal dysphagia. Dig Dis. 1999;17:230–4.
Park YM, Lee SY, Park SW, Kim SH. Role of cancer stem cell in radioresistant head and neck cancer. Auris Nasus Larynx. 2016;43:556–61.
Shirai K, Saitoh JI, Musha A, et al. Clinical outcomes of definitive and postoperative radiotherapy for Stage I–IVB hypopharyngeal cancer. Anticancer Res. 2016;36:6571–8.
Yang L, Chen WK, Guo ZM, Gu MF, Huang HQ, Zhang Q, Yang AK. Long-term survival of induction chemotherapy plus surgery and postoperative radiotherapy in patients with stage IV hypopharyngeal cancer. Anticancer Drugs. 2010;21:872–6.
Laccourreye O, Hans S, Ménard M, Garcia D, Brasnu D, Holsinger FC. Transoral lateral oropharyngectomy for squamous cell carcinoma of the tonsillar region: II. An analysis of the incidence, related variables, and consequences of local recurrence. Arch Otolaryngol Head Neck Surg. 2005;131:592–9.
Weinstein GS, O’Malley BW Jr, Cohen MA, Quon H. Transoral robotic surgery for advanced oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2010;136:1079–85.
Moore EJ, Olsen KD, Kasperbauer JL. Transoral robotic surgery for oropharyngeal squamous cell carcinoma: a prospective study of feasibility and functional outcomes. Laryngoscope. 2009;119:2156–64.
Weinstein GS, O’Malley BW Jr, Cohen MA, Quon H. Transoral robotic surgery for advanced oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2010;136:1079–85.
Haughey BH, Hinni ML, Salassa JR, et al. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: a United States multicenter study. Head Neck. 2011;33:1683–94.
Weinstein GS, O’Malley BW Jr, Hockstein NG. Transoral robotic surgery: supraglottic laryngectomy in a canine model. Laryngoscope. 2005;115:1315–9.
Moore EJ, Henstrom DK, Olsen KD, Kasperbauer JL, McGree ME. Transoral resection of tonsillar squamous cell carcinoma. Laryngoscope. 2009;119:508–15.
DISCLOSURES
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2017_6001_MOESM1_ESM.jpg
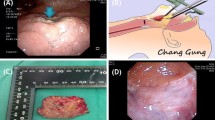
SUPPLEMENTAL FIG. 1 Schematic view of neoadjuvant chemotherapy plus transoral robotic surgery (TORS) for a patient with T3 tonsillar cancer. A The tumor originated from the left tonsil with tongue base invasion. B After three cycles of neoadjuvant chemotherapy, the tumor size was decreased significantly. The primary lesion was initially video-recorded via rigid or flexible endoscopy. C Resection margins (blue line) were mapped based on the pretreatment dimensions (red line) rather than the dimensions of the reduced tumors after neoadjuvant chemotherapy. D The pathologic specimen was analyzed for margin status and other adverse pathologic features. (JPEG 172 kb)
10434_2017_6001_MOESM2_ESM.jpg
SUPPLEMENTAL FIG. 2 Patient with a diagnosis of T4aN3M0 tonsillar cancer. A A huge tumor was observed in the left tonsillar fossa, and a metastatic node more than 6 cm in diameter was evident at level 2 of the left neck. B After three cycles of neoadjuvant chemotherapy, the size of the primary tumor and metastatic node was significantly decreased, and transoral robotic lateral oropharyngectomy was performed based on the initial extent of the disease with modified radical neck dissection. C The pathologic specimen was confirmed to have negative margins. The patient was alive 1 year postoperatively with no evidence of disease (JPEG 122 kb)
Rights and permissions
About this article
Cite this article
Park, Y.M., Jung, C.M., Cha, D. et al. A New Clinical Trial of Neoadjuvant Chemotherapy Combined With Transoral Robotic Surgery and Customized Adjuvant Therapy for Patients With T3 or T4 Oropharyngeal Cancer. Ann Surg Oncol 24, 3424–3429 (2017). https://doi.org/10.1245/s10434-017-6001-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-6001-5