Abstract
Purpose
Receipt of 6 cycles of adjuvant chemotherapy (AC) is standard of care in pancreatic cancer (PC). Neoadjuvant chemotherapy (NAC) is increasingly utilized; however, optimal number of cycles needed alone or in combination with AC remains unknown. We sought to determine the optimal number and sequence of perioperative chemotherapy cycles in PC.
Methods
Single institutional review of all resected PCs from 2008 to 2015. The impact of cumulative number of chemotherapy cycles received (0, 1–5, and ≥6 cycles) and their sequence (NAC, AC, or NAC + AC) on overall survival was evaluated Cox-proportional hazard modeling, using 6 cycles of AC as reference.
Results
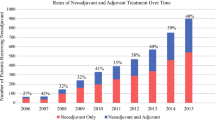
A total of 522 patients were analyzed. Based on sample size distribution, four combinations were evaluated: 0 cycles = 12.1%, 1–5 cycles of combined NAC + AC = 29%, 6 cycles of AC = 25%, and ≥6 cycles of combined NAC + AC = 34%, with corresponding survival. 13.1, 18.5, 37, and 36.8 months. On MVA (P < 0.0001), tumor stage [hazard ratio (HR) 1.35], LNR (HR 4.3), and R1 margins (HR 1.77) were associated with increased hazard of death. Compared with 6 cycles AC, receipt of 0 cycles [HR 3.57, confidence interval (CI) 2.47–5.18] or 1–5 cycles in any combination (HR 2.37, CI 1.73–3.23) was associated with increased hazard of death, whereas receipt of ≥6 cycles in any sequence was associated with optimal and comparable survival (HR 1.07, CI 0.78–1.47).
Conclusions
Receipt of 6 or more perioperative cycles of chemotherapy either as combined neoadjuvant and adjuvant or adjuvant alone may be associated with optimal and comparable survival in resected PC.


Similar content being viewed by others
References
Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10.
Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–77.
Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–81.
Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: Phase III trial of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Ann Surg. 1999;230:776–82; discussion 782-4.
Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs. gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299(9):1019–26.
Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. (epub, ahead of print) 2017.
Valle JW, Palmer D, Jackson R, et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol. 2014;32:504–12.
Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19(1):169–75.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
Aloia TA, Lee JE, Vauthey JN, et al. Delayed recovery after pancreaticoduodenectomy: A major factor impairing the delivery of adjuvant therapy? J Am Coll Surg. 2007;204:347–55.
Tzeng CW, Tran Cao HS, Lee JE, et al. Treatment sequencing for resectable pancreatic cancer: Influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg. 2014;18:16–24; discussion 24-5.
Mayo SC, Gilson MM, Herman JM, et al. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg. 2012;214:33–45.
Wu W, He J, Cameron JL, et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol. 2014;21(9):2873–81.
Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3496–502.
Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3487–95.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Kaplan EL, Meier P. Nonparametric-estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304(10):1073–81.
Bergquist JR, Ivanics T, Shubert CR, et al. Type of resection (Whipple vs. distal) does not affect the national failure to provide post-resection adjuvant chemotherapy in localized pancreatic cancer. Ann Surg Oncol. (epub, ahead of print), 2017.
Wu W, He J, Cameron JL, et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol. 2014;21:2873–81.
Cooper AB, Parmar AD, Riall TS, et al. Does the use of neoadjuvant therapy for pancreatic adenocarcinoma increase postoperative morbidity and mortality rates? J Gastrointest Surg. 2015;19:80–6; discussion 86-7.
Mirkin KA, Hollenbeak CS, Gusani NJ, et al. Trends in utilization of neoadjuvant therapy and short-term outcomes in resected pancreatic cancer. Am J Surg. (epub ahead of print), 2016.
Dimou F, Sineshaw H, Parmar AD, et al. Trends in receipt and timing of multimodality therapy in early-stage pancreatic cancer. J Gastrointest Surg. 2016;20:93–103; discussion 103.
Disclosure
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Epelboym, I., Zenati, M.S., Hamad, A. et al. Analysis of Perioperative Chemotherapy in Resected Pancreatic Cancer: Identifying the Number and Sequence of Chemotherapy Cycles Needed to Optimize Survival. Ann Surg Oncol 24, 2744–2751 (2017). https://doi.org/10.1245/s10434-017-5975-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-5975-3