Abstract
Introduction
In patients with potentially resectable esophageal cancer (EC), the value of endoscopic ultrasonography (EUS) after fluorine-18 labeled fluorodeoxyglucose positron emission tomography with computed tomography (18F-FDG-PET/CT) is questionable. Retrospectively, we assessed the impact of EUS after PET/CT on the given treatment in EC patients.
Methods
During the period 2009–2015, 318 EC patients were staged as T1-4aN0-3M0 with hybrid 18F-FDG-PET/CT or 18F-FDG-PET with CT and EUS if applicable in a nonspecific order. We determined the impact of EUS on the given treatment in 279 patients who also were staged with EUS. EUS had clinical consequences if it changed curability, extent of radiation fields or lymph node resection (AJCC stations 2–5), and when the performed fine-needle aspiration (FNA) provided conclusive information of suspicious lymph node.
Results
EUS had an impact in 80 (28.7%) patients; it changed the radiation field in 63 (22.6%), curability in 5 (1.8%), lymphadenectomy in 48 (17.2%), and FNA was additional in 21 (7.5%). In patients treated with nCRT (n = 194), EUS influenced treatment in 53 (27.3%) patients; in 38 (19.6%) the radiation field changed, in 3 (1.5%) the curability, in 35 (18.0%) the lymphadenectomy, and in 17 (8.8%) FNA was additional. EUS influenced both the extent of radiation field and nodal resection in 31 (16.0%) nCRT patients.
Conclusions
EUS had an impact on the given treatment in approximately 29%. In most patients, the magnitude of EUS found expression in the extent of radiotherapy target volume delineation to upper/high mediastinal lymph nodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
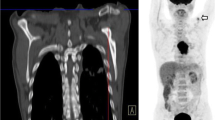
Accurate staging is essential for oncological outcome in patients with potentially curable esophageal cancer for determining the optimal treatment approach and adequate target volume delineation (gross tumor volume of primary tumor and malignant nodes and hence clinical target volume) when radiotherapy is indicated. Fluorine-18 labeled fluorodeoxyglucose positron emission tomography with computed tomography (18F-FDG-PET/CT) is currently the best method to detect distant metastases, whereas endoscopic ultrasonography (EUS) with or without fine-needle aspiration (FNA) is the method of choice to determine the extent and depth-growth of the primary tumor (T-stage) and detection of lymph node metastases (N-stage).1–3
Contradictory results have been reported regarding the additional role of EUS after 18F-FDG-PET, 18F-FDG-PET/CT or CT alone.4–8 Mortensen et al. found that EUS influenced treatment decisions in 34% of the EC patients after CT alone, whereas Findlay et al. found that the risks outweighed the potential benefit of EUS in patients with T2-4a disease on CT.4,5 Some studies showed upfront 18F-FDG-PET followed by EUS as the best predictor of curative resectability and the most cost-effective staging sequence.7,8 However, most studies determined the influence of EUS with questionnaires completed by involved medical specialists and not with the clinical impact or diagnostic position of EUS after 18F-FDG-PET/CT.4,6 Moreover, these studies did not assess the magnitude of EUS on radiotherapy target volume delineation; nowadays most patients receive radiotherapy in a combined curative treatment approach.
In addition, EUS has several limitations, including the invasive character of the procedure with risk of aspiration, perforation, and bleeding, whereas tumor stenosis, which occurs between 20 and 36% of the patients, limits its clinical usage.3,9–11 Precluding unnecessary EUS is therefore patient-friendly in case of a strong suspicion of distant metastases or in case of localized disease on 18F-FDG-PET/CT and reduces the risks of complications and eventually costs.
With 18F-FDG-PET/CT upfront staging sequence, unnecessary primary endoscopic procedures may be prevented, by 18F-FDG-PET/CT guiding of concurrent EUS with fine needle aspiration (FNA) of pathological (non)regional lymph nodes.
The purpose of this study was to determine the clinical impact of EUS after upfront 18F-FDG-PET/CT staging on the given treatment, by determining if EUS had changed radiotherapy target volume delineation, curability, extent of lymph node resection, and whether FNA was of additive value. We also investigated the additional value of EUS on nodal up/downstaging, on the number of lymph nodes suspected for metastasis, and station-specific nodal (N) status.
Patients and Methods
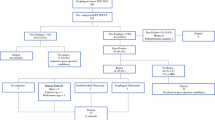
A total of 318 patients were included in this retrospective study, which was performed according to the guidelines of the local Ethical Boards and national rules for retrospective studies. All esophageal cancer patients (n = 388) scheduled for an in onset curative intended treatment (T1-4aN0-3M0), diagnosed at the University Medical Center Groningen (UMCG) or Medical Center Leeuwarden between 2009 and 2015, were eligible for inclusion. All consecutive patients had a pathologically proven adeno- or squamous cell carcinoma, located in the mid or distal esophagus or at the gastroesophageal junction. Excluded were patients treated with endoscopic mucosal resection before (n = 7) or after (n = 3) staging, without 18F-FDG-PET or 18F-FDG-PET/CT scanning (n = 17), without a visible tumor on 18F-FDG-PET/CT (n = 15), or with missing staging data (n = 28).
Methods
First, we determined the number of patients who had a complete or incomplete EUS and those without EUS. To answer the primary research question, all patients with a complete or incomplete EUS were selected. We then assessed if EUS had an impact on the given treatment. Compared with the noninvasive 18F-FDG-PET/CT, EUS had influenced the given treatment if: EUS changed the extent of radiation target volumes (smaller, larger, or both), changed the curability (from incurable based on 18F-FDG-PET/CT to potentially curable), influenced the extent of lymph node dissection (i.e., changed suspicion for lymph node metastasis at AJCC lymph node stations 2–5), or/and when the performed fine-needle aspiration (FNA) provided conclusive information of suspicious lymph node.12 Thereafter, we assessed the clinical impact of EUS on the given treatment in the total patient group (all treatments with curative intent), followed by the neoadjuvant chemoradiotherapy (nCRT), the surgery-alone, and a combined (chemoradiotherapy and definitive radiotherapy (dCRT/dRT) group.
The researchers (JBH & VEMM) assessed the radiotherapy tumor volume (TV) delineations and determined if the EUS findings had changed the radiotherapy target volumes. EUS enlarged the radiotherapy target volume delineation if EUS-FNA had identified new lymph node suspicious for metastases that were located outside of the clinical target volume (CTV: treatment section) based on the 18F-FDG-PET/CT. Lymph nodes were scored by size, shape, echoic pattern, and sharpness of the border. EUS decreased the radiotherapy target volume delineation if initially suspected lymph nodes on the 18F-FDG-PET/CT, located outside of the CTV, were reassessed as negative based on EUS findings and/or confirmed cytologically as FNA-negative. Secondary outcomes included the number of patients with an altered N-stage, with a difference in the number of lymph nodes suspected for metastases, and changes in the localization of suspected lymph nodes.
Staging
Patients were staged according to the 7th TNM classification of the American Joint Committee on Cancer (AJCC) with diagnostic gastroscopy and biopsy, a diagnostic CT thorax/abdomen, 18F-FDG-PET or 18F-FDG-PET/CT, and EUS if possible.13 Depending on the tumor-specific situation, EUS was performed with either a high-frequency (12–20 MHz) linear or radial probes. Esophageal dilatation was not performed routinely in patients with stenosis. The results of all staging methods were discussed in a multidisciplinary tumor board. Abnormalities and non-regional lesions relatively suspect for being a distant metastasis were either proven pathologically under imaging guided biopsies or assessed with additional imaging techniques i.e., magnetic resonance imaging (MRI) or endobronchial ultrasound/FNA.
Treatment
nCRT followed by surgery consisted of carboplatin and paclitaxel according to the CROSS regimen combined with radiotherapy (41.4 Gy/23 fractions).14 dCRT consisted of either carboplatin/paclitaxel or cisplatin and fluorouracil (Cis-5FU) combined with radiotherapy (50.4 Gy).15 dRT commonly consisted of 60 Gy in 30 fractions.
In all patients who received external beam radiotherapy, the gross tumor volume (GTV) was delineated on a planning CT scan, using all additional staging information. The GTV contained both the primary tumor and adjacent lymph nodes. The clinical target volume (CTV) is a margin of 3–3.5 cm in cranial and caudal direction and 1–2 cm transversally. The CTV was adjusted to normal tissue. Suspicious lymph nodes located outside the CTV were radiated separately with 1-cm margin.
Surgery consisted of either a transthoracic, transhiatal, or minimally invasive radical esophagectomy with a two-field lymph node dissection and was normally performed 6–10 weeks after the end of nCRT.
Statistical Analysis
All data were displayed as numbers (percentages). Normally distributed data were displayed as mean (standard deviation). Nonnormally distributed data were displayed as median [interquartile range (IQR)]. Differences in categorical variables were assessed using logistic regression, Chi square test, or likelihood ratio. All data were analyzed using SPSS statistical software, version 22 (SPSS Inc., Chicago, IL).
Results
Table 1 displays the patients’ characteristics, tumor-related, and treatment-related data of the 318 included patients. Of these patients, 250 (78.6%) had an adenocarcinoma and 68 (21.4%) had a squamous cell carcinoma. The localization of the primary tumor was generally in the distal esophagus (n = 221: 69.5%), followed by the gastroesophageal junction (n = 52: 16.4%), and mid esophagus (n = 45: 14.2%). Most patients were treated with curative intent with surgery after nCRT (n = 212: 66.7%), followed by surgery-alone (n = 50: 15.7%), dCRT (n = 29: 9.1%), and dRT (n = 27: 8.5%).
Table 2 displays the endoscopic ultrasonography-related data. EUS was not performed in 39 (12.3%) patients because of stenosis (n = 30/9.4%), patient related reasons (n = 4), and for unknown reasons (n = 5). In total, EUS could be performed in 279 patients: 200 (62.9%) had a complete and 79 (24.8%) an incomplete EUS, because of stenosis (n = 78/24.5%) and patients related reason (n = 1).
Influence of EUS on the Given Treatment
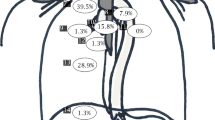
Table 3 displays the additional information that EUS provided compared with upfront 18F-FDG-PET/CT. EUS had influenced the primary treatment in 80 (28.7%) patients. In most patients (n = 63: 22.6%), EUS influenced the extent of radiation target volumes: by increasing the radiotherapy target volume delineation in 45 (16.1%) patients, decreasing these delineation in 17 (6.1%), and both extending and decreasing the target volume in 1 (0.4%) patient. EUS had changed the curability (incurable to potentially curable) in 5 (1.8%) patients, influenced the extent of LN resection (at node level AJCC Lymph node stations 2–5) in 48 (17.2%), and affected treatment decision-making with FNA in 21 patients (7.5%).
Thereafter, we found that histologic tumor type did not influence the effect of EUS on the given treatment (P = 0.614). The location of the primary tumor also influenced the effect of EUS on the given treatment (P = 0.043): midesophageal-located cancers interfered with the treatment in 16 (47.1%), distal EC in 50 (25.3%), and gastroesophageal junction tumors in 14 (29.8%) patients.
In the nCRT group (n = 194), EUS changed the proposed treatment based on upfront 18F-FDG-PET-CT in 53 (27.3%). In 52 (26.8%) patients, it influenced the radiation target volumes, in 3 (1.5%) the curability, in 35 (18.0%) the extent of lymph node dissection, and in 17 (8.8%) FNA added valuable information. Of all patients, in 31 (16.0%) EUS affected the extent of both, the radiation target volumes and the lymph node dissection, usually at AJCC lymph node 2–5, implicating EUS to be valuable in identifying mediastinal lymph node metastases, especially these upper/high lymph nodes.
EUS influenced the treatment in 13 (28.9%) surgery-alone treated patients (n = 45), all by extending the lymph node dissection: EUS identified mediastinal/high lymph node metastasis in 9 (20.0%) patients with 18F-FDG-PET/CT positive lymph nodes, whereas in 4 (8.9%) other patients the 18F-FDG-PET/CT positive lymph nodes turned out to be negative on EUS. In 3 (6.7%) of these 13 patients, FNA was performed and conclusive.
EUS influenced the treatment in 14 (35.0%) patients treated with dCRT or dRT: in 11 (27.5%) it changed the radiation target volumes, in 2 (5.0%) the curability, and in 1 (2.5%) patient FNA was additional. In most of these patients EUS enlarged the radiation target volumes 7 (17.5%), while it was decreased in 4 (10.0%) patients.
Additional Value of EUS
Additional lymph nodes were detected in 150 (53.8%) patients, whereas less suspicious lymph nodes were found in 32 (11.5%). EUS caused N upstaging in 107 (38.4%) and N downstaging in 31 (11.1%) patients. In 77 (27.6%) patients, EUS found suspicious lymph nodes at other nodal stations than with 18F-FDG-PET/CT. In total, 7 (2.5%) patients received a re-EUS examination after primary EUS, because of suspect lymph nodes on the 18F-FDG-PET or CT (n = 3), an unclear outcome of the FNA (n = 3), or an unclear outcome of the EUS itself (n = 1).
Discussion
Accurate staging is of vital importance in the treatment decision-making process and radiotherapy target volume delineation and hence the adjusted correct treatment. The value of EUS after an upfront 18F-FDG-PET/CT scan remains matter or debate. Staging with EUS seems required, because it has the highest sensitivity and accuracy in detecting lymph nodes suspicious for metastatic disease, whereas high regional recurrence rates also suggested the need for a more accurate radiotherapy delineation of involved lymph node stations. In clinical practice, not all patients benefit from EUS because of severe stenosis in approximately 20–36%.11 The present study is the first to determine the influence of EUS on the given treatment with curative intent. We found that EUS influenced treatment in approximately 29% of the patients. Moreover, EUS was especially important for adequate delineation of radiotherapy target volumes, implicating its importance in locoregional control and treatment of esophageal cancer patients with nCRT.
Several studies previously determined the influence of EUS on treatment decision-making, but not on the given treatment itself. Two studies determined the influence of EUS after CT-alone, with a questionnaire, and found that EUS changed the treatment decision in 24–34% of the patients.4,6 A study by Van Zoonen determined that although EUS increased the specificity after CT and ultrasound of the neck, the additional value on determining the surgical resectability was limited.16 Moreover, Findlay et al. found that the risk of EUS in T2-4a patients on CT outweighed the additional information of EUS on T- and N-stage.5 The only study that compared EUS with 18F-FDG-PET/CT was the study by Schreurs et al., which found that 18F-FDG-PET/CT was the best predictor of curability of the resection.8 However, as mentioned, the studies above only determined the influence of EUS on treatment decision making and not on the given treatment itself, whereas detection of metastatic lymph nodes located at some distance of the primary tumor is of fast importance for radiotherapy delineation in the era of nCRT. Currently, the role of EUS in treatment decision making might be limited: In the current treatment paradigm, treatment decision making in T2-4aN0-3M0 esophageal cancer patients is most commonly based on the presence of comorbidities that might not permit nCRT or dCRT. As mentioned, EUS is especially important in patients eligible for nCRT, dCRT, or dRT. Refraining from EUS would have caused inadequate radiotherapy target volumes in approximately 19.6% of the nCRT and 17.5% of the dCRT and dRT patients. In patients eligible for treatment with dCRT and dRT group, this might lead to impaired locoregional control of disease. On the other hand, refraining from EUS would have caused an unnecessary large radiotherapy target volumes in approximately 7% of the patients treated with nCRT and 10% of the dCRT and dRT, which could increase the risk of radiotherapy induced toxicities.
In the future, diffusion-weighted magnetic resonance imaging (DW-MRI) with contrast agents that are taken up by lymph nodes, i.e., Gadofosveset or ultra-small iron particles, might improve the detection of suspicious lymph nodes.17 Moreover, in the coming years proton therapy will become more clinically available, which will decrease the radiation associated (cardiopulmonary) toxicities.18
An important limitation of this study is its retrospective character, which sometimes impedes the exact anatomical information about suspected lymph node metastases, although all lymph node metastases were scored according to the AJCC system by the researchers. The rate of FNA procedures was rather low in present study (n = 58: 20.8%), which may have resulted in false-positive EUS results and probably unnecessary upstaging, because the accuracy of EUS for predicting lymph nodes on echo features in different cancers is approximately 80%.19 A large, prospective study with well-defined FNA of suspected lymph nodes might determine the exact role of EUS in up- and downstaging, although accurate EUS with FNA is time-consuming and should be performed in centralized institute because of its relative complexity. In improving the detection of involved regions with potential harmless imaging modalities, the recent use of DW-MRI seems to be of great importance.
In conclusion, our study determined the additional value of EUS on the given treatment and found that EUS was of added value in approximately 29%. EUS seems especially important for radiotherapy target volume delineation of mediastinal and high mediastinal lymph nodes metastases, implicating its importance in staging esophageal cancer patients planned to be treated with curative intended nCRT. Future, prospective studies with current sophisticated imaging would determine the exact place of EUS-FNA in EC staging.
References
Bruzzi JF, Munden RF, Truong MT, et al. PET/CT of esophageal cancer: its role in clinical management. Radiographics. 2007;27:1635–52.
van Vliet EP, Heijenbrok-Kal MH, Hunink MG, Kuipers EJ, Siersema PD. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer. 2008;98:547–57.
Kelly S, Harris KM, Berry E, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut. 2001;49:534–39.
Mortensen MB, Edwin B, Hunerbein M, Liedman B, Nielsen HO, Hovendal C. Impact of endoscopic ultrasonography (EUS) on surgical decision-making in upper gastrointestinal tract cancer: an international multicenter study. Surg Endosc. 2007;21:431–38.
Findlay JM, Bradley KM, Maile EJ, et al. Pragmatic staging of oesophageal cancer using decision theory involving selective endoscopic ultrasonography, PET and laparoscopy. Br J Surg. 2015;102:1488–499.
Gines A, Fernandez-Esparrach G, Pellise M, Llach-Osendino J, Mata A, Bordas JM. Impact of endoscopic ultrasonography (EUS) and EUS-guided fine-needle aspiration (EUS-FNA) in the management of patients with esophageal cancer. A critical review of the literature. Gastroenterol Hepatol. 2006;29:314–19.
Wallace MB, Nietert PJ, Earle C, et al. An analysis of multiple staging management strategies for carcinoma of the esophagus: computed tomography, endoscopic ultrasound, positron emission tomography, and thoracoscopy/laparoscopy. Ann Thorac Surg. 2002;74:1026–32.
Schreurs LM, Janssens AC, Groen H, et al. Value of EUS in Determining curative resectability in reference to CT and FDG-PET: the optimal sequence in preoperative staging of esophageal cancer? Ann Surg Oncol. 2016;23:1021–28.
Westerterp M, van Westreenen HL, Deutekom M, et al. Patients’ perception of diagnostic tests in the preoperative assessment of esophageal cancer. Patient Prefer Adherence. 2008;2:157–62.
Heeren PA, van Westreenen HL, Geersing GJ, van Dullemen HM, Plukker JT. Influence of tumor characteristics on the accuracy of endoscopic ultrasonography in staging cancer of the esophagus and esophagogastric junction. Endoscopy. 2004;36:966–71.
Catalano MF, Van Dam J, Sivak MV Jr. Malignant esophageal strictures: staging accuracy of endoscopic ultrasonography. Gastrointest Endosc. 1995;41:535–39.
Edge SB. AJCC cancer staging handbook. 2010.
American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. New York: Springer; 2009.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–84.
Honing J, Smit JK, Muijs CT, et al. A comparison of carboplatin and paclitaxel with cisplatinum and 5-fluorouracil in definitive chemoradiation in esophageal cancer patients. Ann Oncol. 2014;25:638–43.
van Zoonen M, van Oijen MG, van Leeuwen MS, van Hillegersberg R, Siersema PD, Vleggaar FP. Low impact of staging EUS for determining surgical resectability in esophageal cancer. Surg Endosc. 2012;26:2828–34.
Heijnen LA, Lambregts DM, Martens MH, et al. Performance of gadofosveset-enhanced MRI for staging rectal cancer nodes: can the initial promising results be reproduced? Eur Radiol. 2014;24:371–79.
Verma V, Lin SH, Simone CB 2nd, Mehta MP. Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: a systematic review. J Gastrointest Oncol. 2016;7:644–64.
Chen VK, Eloubeidi MA. Endoscopic ultrasound-guided fine needle aspiration is superior to lymph node echofeatures: a prospective evaluation of mediastinal and peri-intestinal lymphadenopathy. Am J Gastroenterol. 2004;99:628–33.
Disclosure
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
J.B. Hulshoff control over data.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hulshoff, J.B., Mul, V.E.M., de Boer, H.E.M. et al. Impact of Endoscopic Ultrasonography on 18F-FDG-PET/CT Upfront Towards Patient Specific Esophageal Cancer Treatment. Ann Surg Oncol 24, 1828–1834 (2017). https://doi.org/10.1245/s10434-017-5835-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-5835-1