Abstract
Background
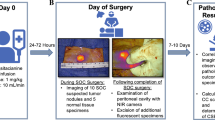
Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) is a treatment option for peritoneal surface malignancies. The ability to detect microscopic foci of peritoneal metastasis intraoperatively may ensure the completeness of cytoreduction. In this study, we evaluated the suitability of a hand-held cathepsin-based fluorescent imaging system for intraoperative detection of appendiceal and colorectal peritoneal metastasis.
Methods
Peritoneal tumors and normal peritoneal tissues were collected from patients with appendiceal and colorectal peritoneal metastasis. Expression of different cathepsins (CTS-B, -D, -F, -G, -K, -L, -O, and -S) was determined by quantitative RT-PCR and immunohistochemistry. The hand-held cathepsin-based fluorescent imaging system was used to detect peritoneal xenografts derived from human colon cancer cells (HT29, LoVo and HCT116) in nu/nu mice.
Results
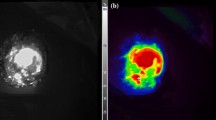
While the expression levels of CTS-B, -D, -L, and -S could be higher in peritoneal tumors than normal peritoneum with a median (range) of 6.1 (2.9–25.8), 2.0 (1.0–15.8), 1.4 (0.8–7.0), and 2.1 (1.6–13.9) folds by quantitative RT-PCR, respectively, CTS-B was consistently the major contributor of the overall cathepsin expression in appendiceal and colonic peritoneal tumors, including adenocarcinomas and low-grade appendiceal mucinous neoplasms. Using peritoneal xenograft mouse models, small barely visible colonic peritoneal tumors (<2.5 mm in maximum diameter) could be detected by the hand-held cathepsin-based fluorescent imaging system.
Conclusions
Because cathepsin expression is higher in peritoneal tumors than underlying peritoneum, the hand-held cathepsin-based fluorescent imaging system could be useful for intraoperative detection of microscopic peritoneal metastasis during CRS-HIPEC and clinical trial is warranted.




Similar content being viewed by others
References
Valle M, Federici O, Garofalo A. Patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy, and role of laparoscopy in diagnosis, staging, and treatment. Surg Oncol Clin N Am. 2012;21:515–32.
Votanopoulos KI, Shen P, Stewart IV JH, Levine EA. Current status and future directions in appendiceal cancer with peritoneal dissemination. Surg Oncol Clin N Am. 2012;21:599–610.
Elias D, Quenet F, Goere D. Current status and future directions in the treatment of peritoneal dissemination from colorectal carcinoma. Surg Oncol Clin N Am. 2012;21:611–24.
Glockzin G, Piso P. Current status and future directions in gastric cancer with peritoneal dissemination. Surg Oncol Clin N Am. 2012;21:625–34.
Helm CW. Current status and future directions of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of ovarian cancer. Surg Oncol Clin N Am. 2012;21:645–64.
Whitley MJ, Cardona DM, Lazarides AL, et al. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci Transl Med. 2016:8:320ra4.
Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–75.
Mito JK, Ferrer JM, Brigman BE, et al. Intraoperative detection and removal of microscopic residual sarcoma using wide-field imaging. Cancer. 2012;118;5320–30.
Eward WC, Mito JK, Eward CA, et al. A novel imaging system permits real-time in vivo tumor bed assessment after resection of naturally occurring sarcomas in dogs. Clin Orthop Relat Res. 2013;471:834–42.
Cuneo KC, Mito JK, Javid MP, et al. Imaging primary mouse sarcomas after radiation therapy using cathepsin-activatable fluorescent imaging agents. Int J Radiat Oncol Biol Phys. 2013;86:136–42.
Tan G, Peng Z, Lu J, Tang F. Cathepsins mediate tumor metastasis. World J Biol Chem. 2013;4:91–101.
Chauhan SS, Goldstein LJ, Gottesman MM. Expression of cathepsin L in human tumors. Cancer Res. 1991;51:1478–81.
Shuja S, Sheahan K, Murnane MJ. Cysteine endopeptidase activity levels in normal human tissues, colorectal adenomas and carcinomas. Int J Cancer. 1991;49:341–6.
Campo E, Munoz J, Miquel R, et al. Cathepsin B expression in colorectal carcinomas correlates with tumor progression and shortened patient survival. Am J Pathol. 1994;145:301.
Adenis A, Huet G, Zerimech F, Hecquet B, Balduyck M, Peyrat JP. Cathepsin B, L, D activities in colorectal carcinomas: relationship with clinic-pathological parameters. Cancer Lett. 1995;96:267–75.
Mayer A, Fritz E, Fortelny R, Kofler K, Ludwig H. Immunohistochemical evaluation of cathepsin D expression in colorectal cancer. Cancer Invest. 1997;15:106–110.
Gormley JA, Hegarty SM, O’Grady A, et al. The role of Cathepsin S as a marker of prognosis and predictor of chemotherapy benefit in adjuvant CRC: a pilot study. Br J Cancer. 2011;105:1487–94.
Jacob F, Guertier R, Naim S, et al. Careful selection of reference genes is required for reliable performance of RT-qPCR in human normal and cancer cell lines. PLoS ONE. 2013;8:e59180.
Sulpizio S, Franceschini N, Piattelli A, Di Sebastiano P, Innocenti P, Selvaggi F. Cathepsins and pancreatic cancer: the 2012 update. Pancreatology. 2012;12:395–401.
Tan GJ, Peng ZK, Lu JP, Tang FQ. Cathepsins mediate tumor metastasis. World J Biol Chem. 2013;4:91–101.
Olson OC, Joyce JA. Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat Rev Cancer. 2015;15:712–29.
Aggarwal N, Sloane BF. Cathepsin B: multiple roles in cancer. Proteomics Clin Appl. 2014;8:427-37.
Pleijhuis RG, Langhout GC, Helfrich W, et al. Near-infrared fluorescence (NIRF) imaging in breast-conserving surgery: assessing intraoperative techniques in tissue-simulating breast phantoms. Eur J Surg Oncol. 2011;37:32–9.
Stoffels I, Dissemond J, Pöppel T, Schadendorf D, Klode J. Intraoperative fluorescence imaging for sentinel lymph node detection: prospective clinical trial to compare the usefulness of indocyanine green vs technetium Tc 99m for identification of sentinel lymph nodes. JAMA Surg. 2015;150:617–23.
Noura S, Ohue M, Seki Y, et al. Feasibility of a lateral region sentinel node biopsy of lower rectal cancer guided by indocyanine green using a near-infrared camera system. Ann Surg Oncol. 2010;17:144–51.
Handgraaf HJ, Boogerd LS, Verbeek FP, et al. Intraoperative fluorescence imaging to localize tumors and sentinel lymph nodes in rectal cancer. Minim Invasive Ther Allied Technol. 2016;25:48–53.
Liberale G, Vankerckhove S, Galdon MG, Donckier V, Larsimont D, Bourgeois P. Fluorescence imaging after intraoperative intravenous injection of indocyanine green for detection of lymph node metastases in colorectal cancer. Eur J Surg Oncol. 2015;41:1256–60.
Kabuto M, Kubota T, Kobayashi H, et al. Experimental and clinical study of detection of glioma at surgery using fluorescent imaging by a surgical microscope after fluorescein administration. Neurol Res. 1997;19:9–16.
Liberale G, Vankerckhove S, Galdon MG, et al. Fluorescence imaging after indocyanine green injection for detection of peritoneal metastases in patients undergoing cytoreductive surgery for peritoneal carcinomatosis from colorectal cancer. Ann Surg. 2016.
Satou S, Ishizawa T, Masuda K, et al. Indocyanine green fluorescent imaging for detecting extrahepatic metastasis of hepatocellular carcinoma. J Gastroenterol. 2013;48:1136–43.
Acknowledgement
The authors thank Lumicell, Inc. for providing the LUM015 imaging agent and the LUM Imaging Device.
Authors’ Contribution
CH.F.C. contributed to the conception, design, data collection, data analysis, and article preparation. L.F.L. contributed to the design, data collection, data analysis, and article review. I.F. contributed to data analysis and article review. J.C.C. contributed to the conception and article review.
Disclosure
CH.F.C., L.F.L., I.F., and J.C.C. report neither any disclosure nor any financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Carlos H. F. Chan and Lukas F. Liesenfeld have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chan, C.H.F., Liesenfeld, L.F., Ferreiro-Neira, I. et al. Preclinical Evaluation of Cathepsin-Based Fluorescent Imaging System for Cytoreductive Surgery. Ann Surg Oncol 24, 931–938 (2017). https://doi.org/10.1245/s10434-016-5690-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5690-5