Abstract
Background
Advantages of neoadjuvant chemotherapy combined with monoclonal antibodies for treating patients with resectable colorectal cancer liver metastasis (CLM) have not been established. The purpose of this study was to evaluate the efficacy and safety of oxaliplatin-based regimen (FOLFOX or XELOX) plus monoclonal antibodies (cetuximab or bevacizumab) treatment in patients with resectable CLM.
Methods
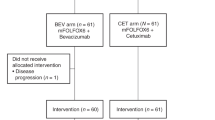
A single-arm, open-label, multicenter, phase II trial was conducted for patients aged ≥ 20 years with resectable and untreated CLM. Patients received preoperative FOLFOX (6 cycles) or XELOX (4 cycles). Cetuximab or bevacizumab was administered to patients with wild-type or mutated KRAS codons 12 and 13, respectively. The primary endpoint was progression-free survival (PFS).
Results
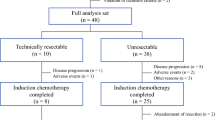
Between January 2010 and June 2012, 47 patients were enrolled from 12 institutions. Wild-type or mutant KRAS sequences were examined in 32 and 15 patients, respectively. Twenty-one (45 %) patients experienced Grades 3/4 adverse events, and 55 % of all patients responded to therapy. The sizes of tumors of patients in the wild-type KRAS group were significantly reduced compared with those of the mutant KRAS group. The overall rates of liver resection and postoperative morbidity were 83 and 14 %, respectively, and the median PFS was 15.6 months. The median PFS times of the KRAS wild-type and mutant groups were 22.5 months and 10.5 months, respectively.
Conclusions
Neoadjuvant therapy using FOLFOX/XELOX combined with monoclonal antibodies did not improve PFS, although it was administered safely and had less adverse effects after liver resection.


Similar content being viewed by others
References
Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502.
Abdalla EK. Resection of colorectal liver metastases. J Gastrointest Surg. 2011;15:416–9.
Bonney GK, Coldham C, Adam R, et al. Role of neoadjuvant chemotherapy in resectable synchronous colorectal liver metastasis; An international multi-center data analysis using LiverMetSurvey. J Surg Oncol. 2015;111:716–24.
de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–8.
Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–48.
Kemeny MM, Adak S, Gray B, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy–an intergroup study. J Clin Oncol. 2002;20:1499–505.
Portier G, Elias D, Bouche O, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976–82.
Kanda M, Kodera Y, Sakamoto J. Updated evidence on adjuvant treatments for gastric cancer. Expert Rev Gastroenterol Hepatol. 2015:1–12.
Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–16.
Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–15.
Primrose J, Falk S, Finch-Jones M, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15:601–11.
Iwamoto S, Hazama S, Kato T, et al. Multicenter phase II study of second-line cetuximab plus folinic acid/5-fluorouracil/irinotecan (FOLFIRI) in KRAS wild-type metastatic colorectal cancer: the FLIER study. Anticancer Res. 2014;34:1967–73.
Munemoto Y, Kanda M, Ishibashi K, et al. Capecitabine and oxaliplatin combined with bevacizumab are feasible for treating selected Japanese patients at least 75 years of age with metastatic colorectal cancer. BMC Cancer. 2015;15:786.
Kanda M, Shimizu D, Fujii T, et al. Function and diagnostic value of Anosmin-1 in gastric cancer progression. Int J Cancer. 2016;138:721–30.
Kanda M, Shimizu D, Tanaka H, et al. Metastatic pathway-specific transcriptome analysis identifies MFSD4 as a putative tumor suppressor and biomarker for hepatic metastasis in patients with gastric cancer. Oncotarget. 2016;7:13667–79.
Benoist S, Nordlinger B. The role of preoperative chemotherapy in patients with resectable colorectal liver metastases. Ann Surg Oncol. 2009;16:2385–90.
Wong R, Cunningham D, Barbachano Y, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol. 2011;22:2042–8.
Khan K, Wale A, Brown G, Chau I. Colorectal cancer with liver metastases: neoadjuvant chemotherapy, surgical resection first or palliation alone? World J Gastroenterol. 2014;20:12391–406.
Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9.
Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17.
Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47.
Kanda M, Nomoto S, Oya H, et al. The Expression of Melanoma-Associated Antigen D2 Both in Surgically Resected and Serum Samples Serves as Clinically Relevant Biomarker of Gastric Cancer Progression. Ann Surg Oncol. 2016;23 Suppl 2:214–21.
Yoshioka Y, Uehara K, Ebata T, et al. Postoperative complications following neoadjuvant bevacizumab treatment for advanced colorectal cancer. Surg Today. 2014;44:1300–6.
Mahfud M, Breitenstein S, El-Badry AM, et al. Impact of preoperative bevacizumab on complications after resection of colorectal liver metastases: case-matched control study. World J Surg. 2010;34:92–100.
Fernandez-Martos C, Brown G, Estevan R, et al. Preoperative chemotherapy in patients with intermediate-risk rectal adenocarcinoma selected by high-resolution magnetic resonance imaging: the GEMCAD 0801 Phase II Multicenter Trial. Oncologist. 2014;19:1042–3.
Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513–8.
Castagnola P, Giaretti W. Mutant KRAS, chromosomal instability and prognosis in colorectal cancer. Biochim Biophys Acta. 2005;1756:115–25.
Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535–46.
Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–14.
Bokemeyer C, Kohne CH, Ciardiello F, et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer. 2015;51:1243–52.
Patterson SD, Cohen N, Karnoub M, et al. Prospective-retrospective biomarker analysis for regulatory consideration: white paper from the industry pharmacogenomics working group. Pharmacogenomics. 2011;12:939–51.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Junichi Sakamoto serves as an advisor to Takeda Pharmaceutical Company Limited and received lecture fees from Tsumura Co., Ltd.
Additional information
Masato Kataoka and Mitsuro Kanda have contributed equally to this work.
An erratum to this article is available at http://dx.doi.org/10.1245/s10434-016-5593-5.
Rights and permissions
About this article
Cite this article
Kataoka, M., Kanda, M., Ishigure, K. et al. The COMET Open-label Phase II Study of Neoadjuvant FOLFOX or XELOX Treatment Combined with Molecular Targeting Monoclonal Antibodies in Patients with Resectable Liver Metastasis of Colorectal Cancer. Ann Surg Oncol 24, 546–553 (2017). https://doi.org/10.1245/s10434-016-5557-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5557-9