Abstract
Background
Several studies have reported a high rate of RHOA mutations in the Lauren diffuse-type gastric adenocarcinoma (GA) but not in intestinal-type GA. The aim of this study was to determine if RhoA activity is prognostic for overall survival (OS) in patients with resectable GA.
Methods
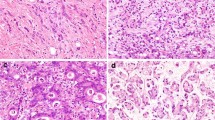
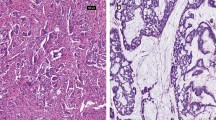
Retrospective review was performed on a prospective database of GA patients who underwent potentially curative resection between 2003 and 2012 at a single institution. Tissue microarrays were constructed from surgical specimens and analyzed for phosphorylated RhoA, a marker of inactive RhoA signaling. OS was estimated by the Kaplan–Meier method, and multivariate analysis was performed by Cox proportional hazards regression modeling.
Results
One hundred thirty-six patients with diffuse-type GA and 129 patients with intestinal-type GA were examined. Compared to intestinal-type GA, diffuse-type GA tumors were significantly associated with increased tumor size and advanced tumor, node, metastasis (TNM) classification system stage. In patients with diffuse-type GA, high RhoA activity was associated with significantly worse OS when compared to low RhoA activity (5-year OS 52.5 vs. 81.0 %, p = 0.017). This difference in OS was not observed in patients with intestinal-type GA (5-year OS 83.9 vs. 81.6 %, p = 0.766). On multivariate analysis of diffuse-type GA patients, high RhoA activity was an independent negative prognostic factor for OS (hazard ratio 2.38, 95 % confidence interval 1.07–5.28).
Conclusions
Increased RhoA activity is predictive of worse OS in patients with diffuse-type GA who undergo potentially curative surgical resection. Along with findings from genomic studies, these results suggest RhoA may be a novel therapeutic target in diffuse-type GA.


Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Kumar V, Abbas AK, Aster JC. Robbins and Cotran pathologic basis of disease. 9th ed. 2015.
Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–62.
Pozzo C, Barone C. Is there an optimal chemotherapy regimen for the treatment of advanced gastric cancer that will provide a platform for the introduction of new biological agents? Oncologist. 2008;13:794–806.
Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46.
Cervantes A, Roda D, Tarazona N, Rosello S, Perez-Fidalgo JA. Current questions for the treatment of advanced gastric cancer. Cancer Treat Rev. 2013;39:60–7.
Lynch HT, Grady W, Suriano G, Huntsman D. Gastric cancer: new genetic developments. J Surg Oncol. 2005;90:114–33.
Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49.
Bosman FT. World Health Organization; International Agency for Research on Cancer. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer; 2010.
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–9.
Wang K, Yuen ST, Xu J, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–82.
Kakiuchi M, Nishizawa T, Ueda H, et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet. 2014;46:583–7.
Guan R, Xu X, Chen M, et al. Advances in the studies of roles of Rho/Rho-kinase in diseases and the development of its inhibitors. Eur J Med Chem. 2013;70:613–22.
Thumkeo D, Watanabe S, Narumiya S. Physiological roles of Rho and Rho effectors in mammals. Eur J Cell Biol. 2013;92:303–15.
Su XJ, Tang ZF, Li Q, et al. [Expression and significance of RhoA and NF-KappaB in human gastric carcinoma]. Zhonghua Zhong Liu Za Zhi. 2011;33:276–9.
Siewert JR, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232:353–61.
Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–9.
Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–7.
DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–63.
Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–81.
Cox D. Regression models and life-tables. J R Stat Soc. 1972;34:187–220.
Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2003;284:L972–80.
Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 1996;15:510–9.
Liu M, Bi F, Zhou X, Zheng Y. Rho GTPase regulation by miRNAs and covalent modifications. Trends Cell Biol. 2012;22:365–73.
Tan IB, Ivanova T, Lim KH, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141:476–85.
Lei Z, Tan IB, Das K, et al. Identification of molecular subtypes of gastric cancer with different responses to PI3-kinase inhibitors and 5-fluorouracil. Gastroenterology. 2013;145:554–65.
Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–56.
Maeda M, Ushijima T. RHOA mutation may be associated with diffuse-type gastric cancer progression, but is it gain or loss? Gastric Cancer. In press.
Kantak SS, Kramer RH. E-cadherin regulates anchorage-independent growth and survival in oral squamous cell carcinoma cells. J Biol Chem. 1998;273:16953–61.
Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009;1796:91–8.
Leve F, Morgado-Diaz JA. Rho GTPase signaling in the development of colorectal cancer. J Cell Biochem. 2012;113:2549–59.
Orgaz JL, Herraiz C, Sanz-Moreno V. Rho GTPases modulate malignant transformation of tumor cells. Small GTPases. 2014;5:e29019.
Takami Y, Higashi M, Kumagai S, et al. The activity of RhoA is correlated with lymph node metastasis in human colorectal cancer. Dig Dis Sci. 2008;53:467–73.
Pan Y, Bi F, Liu N, et al. Expression of seven main Rho family members in gastric carcinoma. Biochem Biophys Res Commun. 2004;315:686–91.
Acknowledgment
Funded in part through National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, K.K., Cho, SJ., Yoon, C. et al. Increased RhoA Activity Predicts Worse Overall Survival in Patients Undergoing Surgical Resection for Lauren Diffuse-Type Gastric Adenocarcinoma. Ann Surg Oncol 23, 4238–4246 (2016). https://doi.org/10.1245/s10434-016-5357-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5357-2