Abstract
Background
This study aimed to compare the type and extent of surgery in patients with screen-detected and interval cancers after blinded or nonblinded double-reading of screening mammograms.
Methods
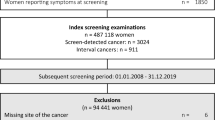
The study investigated a consecutive series of screens double-read in either a blinded (n = 44,491) or nonblinded (n = 42,996) fashion between 2009 and 2011. During a 2 year follow-up period, the radiology reports, surgical correspondence, and pathology reports of all the screen-detected and interval cancers were collected.
Results
Screen-detected breast cancer was diagnosed for 325 women at blinded and 284 women at nonblinded double-reading. The majority of the women were treated by breast-conserving surgery (BCS) at both reading strategies (78.2 vs. 81.7 %; p = 0.51). Larger total resection volumes were observed at BCS for ductal carcinoma in situ (DCIS) treatment for patients after blinded double-reading (p = 0.005). The proportions of positive resection margins after BCS were comparable for patients with DCIS (p = 0.81) or invasive screen-detected cancers (p = 0.38) for the two reading strategies. A total of 158 interval cancers were diagnosed. The proportions of patients treated with BCS were comparable for the two reading strategies (p = 0.42). The total resection volume (p = 0.13) and the proportion of positive resection margins after BCS (p = 0.32) for invasive interval cancer were comparable for the two cohorts. The BCS rate was higher for women after nonblinded double-reading (p = 0.04).
Conclusions
Blinded and nonblinded double-reading yielded comparable surgical treatments for women with screen-detected or interval breast cancer except for larger total resection volumes at BCS for screen-detected DCIS and a higher BCS rate for interval cancers at nonblinded double-reading.
Similar content being viewed by others
References
Kalager M, Zelen M, Langmark F, Adami HO. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363:1203–10.
van Schoor G, Moss SM, Otten JD, et al. Increasingly strong reduction in breast cancer mortality due to screening. Br J Cancer. 2011;104:910–14.
Burton RC, Bell RJ, Thiagarajah G, Stevenson C. Adjuvant therapy, not mammographic screening, accounts for most of the observed breast cancer specific mortality reductions in Australian women since the national screening program began in 1991. Br Cancer Res Treat. 2012;131:949–55.
Mandelblatt J, van Ravesteyn N, Schechter C, et al. Which strategies reduce breast cancer mortality most? Collaborative modeling of optimal screening, treatment, and obesity prevention. Cancer. 2013;119:2541–8.
Weedon-Fekjaer H, Romundstad PR, Vatten LJ. Modern mammography screening and breast cancer mortality: population study. BMJ. 2014;348:g3701.
Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA. 2014;311:1327–35.
Cady B, Stone MD, Schuler JG, Thakur R, Wanner MA, Lavin PT. The new era in breast cancer: invasion, size, and nodal involvement dramatically decreasing as a result of mammographic screening. Arch Surg. 1996;131:301–8.
Veronesi U, Banfi A, Salvadori B, et al. Breast conservation is the treatment of choice in small breast cancer: long-term results of a randomized trial. Eur J Cancer. 1990;26:668–70.
Samnakay N, Tinning J, Ives A, et al. Rates for mastectomy are lower in women attending a breast-screening programme. ANZ J Surg. 2005;75:936–9.
Duijm LE, Louwman MW, Groenewoud JH, van de Poll-Franse LV, Fracheboud J, Coebergh JW. Interobserver variability in mammography screening and effect of type and number of readers on screening outcome. Br J Cancer. 2009;100:901–7.
Gur D, Sumkin JH, Hardesty LA, et al. Recall and detection rates in screening mammography. Cancer. 2004;100:1590–4.
Klompenhouwer EG, Voogd AC, den Heeten GJ, et al. Blinded double-reading yields a higher programme sensitivity than nonblinded double-reading at digital screening mammography: a prospected population-based study in the south of The Netherlands. Eur J Cancer. 2015;51:391–9.
Weber RJ, Klompenhouwer EG, Voogd AC, Strobbe LJ, Broeders MJ, Duijm LE. Comparison of the diagnostic workup of women referred at nonblinded or blinded double-reading in a population-based screening mammography programme in the south of the Netherlands. Br J Cancer. 2015;113:1094–8.
Duijm LE, Groenewoud JH, Hendriks JH, de Koning HJ. Independent double-reading of screening mammograms in The Netherlands: effect of arbitration following reader disagreements. Radiology. 2004;231:564–70.
Liberman L, Menell JH. Breast imaging reporting and data system (BI-RADS). Radiol Clin North Am. 2002;40:409–30.
Morrow M. Breast conservation and negative margins: how much is enough? Breast. 2009;18(Suppl 3):S84–6.
Krekel N, Zonderhuis B, Muller S, et al. Excessive resections in breast-conserving surgery: a retrospective multicentre study. Breast J. 2011;17:602–9.
van Steenbergen LN, Voogd AC, Roukema JA, et al. Time trends and inter-hospital variation in treatment and axillary staging of patients with ductal carcinoma in situ of the breast in the era of screening in Southern Netherlands. Breast. 2014;23:63–8.
Ponti A, Lynge E, James T, et al. International variation in management of screen-detected ductal carcinoma in situ of the breast. Eur J Cancer. 2014;50:2695–704.
Schouten van der Velden AP, Van Dijck JA, Wobbes T. Variations in treatment of ductal carcinoma in situ of the breast: a population-based study in the East Netherlands. Eur J Surg Oncol. 2007;33:424–9.
Weber RJ, Nederend J, Voogd AC, Strobbe LJ, Duijm LE. Screening outcome and surgical treatment during and after the transition from screen-film to digital screening mammography in the south of The Netherlands. Int J Cancer. 2015;137:135–43.
Hofvind S, Skaane P, Elmore JG, Sebuodegard S, Hoff SR, Lee CI. Mammographic performance in a population-based screening program: before, during, and after the transition from screen-film to full-field digital mammography. Radiology. 2014;272:52–62.
Cochrane RA, Valasiadou P, Wilson AR, Al-Ghazal SK, Macmillan RD. Cosmesis and satisfaction after breast-conserving surgery correlates with the percentage of breast volume excised. Br J Surg. 2003;90:1505–9.
Ernst MF, Voogd AC, Coebergh JW, Repelaer van Driel OJ, Roukema JA. The introduction of mammographical screening has had little effect on the trend in breast-conserving surgery: a population-based study in Southeast Netherlands. Eur J Cancer. 2001;37:2435–40.
Christiansen P, Vejborg I, Kroman N, et al. Position paper: breast cancer screening, diagnosis, and treatment in Denmark. Acta Oncol. 2014;53:433–44.
Nederend J, Duijm LE, Louwman MW, Roumen RM, Jansen FH, Voogd AC. Trends in surgery for screen-detected and interval breast cancers in a national screening programme. Br J Surg. 2014;101:949–58.
Smitt MC, Horst K. Association of clinical and pathologic variables with lumpectomy surgical margin status after preoperative diagnosis or excisional biopsy of invasive breast cancer. Ann Surg Oncol. 2007;14:1040–4.
Aziz D, Rawlinson E, Narod SA, et al. The role of reexcision for positive margins in optimizing local disease control after breast-conserving surgery for cancer. Breast J. 2006;12:331–7.
Chagpar AB, Martin RC II, Hagendoorn LJ, Chao C, McMasters KM. Lumpectomy margins are affected by tumor size and histologic subtype but not by biopsy technique. Am J Surg. 2004;188:399–402.
Zork NM, Komenaka IK, Pennington RE Jr, et al. The effect of dedicated breast surgeons on the short-term outcomes in breast cancer. Ann Surg. 2008;248:280–5.
Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680–5.
Garcia-Fernandez A, Chabrera C, Garcia-Font M, et al. A study comparing two consecutive historical periods in breast cancer with a focus on surgical treatment, loco-regional recurrence, distant metastases, and mortality. Clin Translat Oncol. 2015;17:296–305.
Liebregts ME, van Riet YE, Nieuwenhuijzen GA, Rutten HJ, Duijm LE, Voogd AC. Patterns and determinants of surgical management of screen detected breast cancer in the South-East Netherlands. Breast. 2013;22:713–7.
Al-Ghazal SK, Blamey RW, Stewart J, Morgan AA. The cosmetic outcome in early breast cancer treated with breast conservation. Eur J Surg Oncol. 1999;25:566–70.
Wazer DE, DiPetrillo T, Schmidt-Ullrich R, et al. Factors influencing cosmetic outcome and complication risk after conservative surgery and radiotherapy for early-stage breast carcinoma. J Clin Oncol. 1992;10:356–63.
Smith-Bindman R, Chu PW, Miglioretti DL, et al. Comparison of screening mammography in the United States and the United kingdom. JAMA. 2003;290:2129–37.
Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773–83.
Lewin JM, D’Orsi CJ, Hendrick RE, et al. Clinical comparison of full-field digital mammography and screen-film mammography for detection of breast cancer. AJR Am J Roentgenol. 2002;179:671–7.
Domingo L, Romero A, Belvis F, et al. Differences in radiological patterns, tumour characteristics, and diagnostic precision between digital mammography and screen-film mammography in four breast cancer screening programmes in Spain. Eur Radiol. 2011;21:2020–8.
Juel IM, Skaane P, Hoff SR, Johannessen G, Hofvind S. Screen-film mammography versus full-field digital mammography in a population-based screening program: the Sogn and Fjordane study. Acta Radiol. 2010;51:962–8.
Houssami N, Macaskill P, Bernardi D, et al. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading: evidence to guide future screening strategies. Eur J Cancer. 2014;50:1799–807.
Bargallo X, Santamaria G, Del Amo M, et al. Single-reading with computer-aided detection performed by selected radiologists in a breast cancer screening program. Eur J Radiol. 2014;83:2019–23.
Clarke-Pearson EM, Jacobson AF, Boolbol SK, et al. Quality assurance initiative at one institution for minimally invasive breast biopsy as the initial diagnostic technique. J Am Coll Surg. 2009;208:75–8.
Gutwein LG, Ang DN, Liu H, et al. Utilization of minimally invasive breast biopsy for the evaluation of suspicious breast lesions. Am J Surg. 2011;202:127–32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Weber, R.J.P., van Bommel, R.M.G., Setz-Pels, W. et al. Type and Extent of Surgery for Screen-Detected and Interval Cancers at Blinded Versus Nonblinded Double-Reading in a Population-Based Screening Mammography Program. Ann Surg Oncol 23, 3822–3830 (2016). https://doi.org/10.1245/s10434-016-5295-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5295-z