Abstract
Background
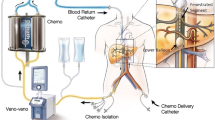
This report describes the outcomes and long-term follow-up data from all isolated hepatic perfusions (IHPs) performed at a single institution in Sweden between the years 1989 and 2013 for patients with isolated uveal melanoma metastases.
Methods
A total of 68 patients (median age, 61 years) were treated consecutively at Sahlgrenska University Hospital. Of the 68 patients, 67 % had fewer than 10 tumors. The median diameter of the largest lesion was 2.5 cm. The patients underwent IHP with either melphalan alone or the addition of either tumor necrosis factor-alpha or cisplatin. The response was assessed after 8–12 weeks by computed tomography or magnetic resonance imaging.
Results
The overall response rate was 67 and 20 % of the patients had a complete response. The median times to local and systemic progression were respectively 10 and 14 months. The prognostic factors for time to local recurrence were response and number of tumors. The median survival time was 22 months. The prognostic factors for survival were response, largest tumor diameter, and number of tumors. Five patients (7 %) died within 30 days, and six patients (9 %) experienced major complications (Clavien–Dindo 3/4).
Conclusions
Isolated hepatic perfusion is a treatment option with high response rates and tolerable mortality and morbidity. Whether IHP has a survival benefit compared with other treatment options currently is being investigated in a randomized trial.

Similar content being viewed by others
References
Bergman L, Seregard S, Nilsson B, Ringborg U, Lundell G, Ragnarsson-Olding B. Incidence of uveal melanoma in Sweden from 1960 to 1998. Invest Ophthalmol Vis Sci. 2002;43:2579–83.
Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123:1639–43.
Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44:4651–9.
Rietschel P, Panageas KS, Hanlon C, Patel A, Abramson DH, Chapman PB. Variates of survival in metastatic uveal melanoma. J Clin Oncol. 2005;23:8076–80.
Gragoudas ES, Egan KM, Seddon JM, et al. Survival of patients with metastases from uveal melanoma. Ophthalmology. 1991;98:383–9; discussion 390.
Kivela T, Suciu S, Hansson J, et al. Bleomycin, vincristine, lomustine, and dacarbazine (BOLD) in combination with recombinant interferon alpha-2b for metastatic uveal melanoma. Eur J Cancer. 2003;39:1115–20.
Carvajal RD, Sosman JA, Quevedo JF, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA. 2014;311:2397–405.
Luke JJ, Callahan MK, Postow MA, et al. Clinical activity of ipilimumab for metastatic uveal melanoma: a retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer. 2013;119:3687–95.
Mariani P, Piperno-Neumann S, Servois V, et al. Surgical management of liver metastases from uveal melanoma: 16 years’ experience at the Institut Curie. Eur J Surg Oncol. 2009;35:1192–7.
Leyvraz S, Piperno-Neumann S, Suciu S, et al. Hepatic intraarterial versus intravenous fotemustine in patients with liver metastases from uveal melanoma (EORTC 18021): a multicentric randomized trial. Ann Oncol. 2014;25:742–6.
Valpione S, Aliberti C, Parrozzani R, et al. A retrospective analysis of 141 patients with liver metastases from uveal melanoma: a two-cohort study comparing transarterial chemoembolization with CPT-11 charged microbeads and historical treatments. Melanoma Res. 2015;25:164–8.
Gonsalves CF, Eschelman DJ, Thornburg B, Frangos A, Sato T. Uveal melanoma metastatic to the liver: chemoembolization with 1,3-bis-(2-chloroethyl)-1-nitrosourea. AJR Am J Roentgenol. 2015;205:1–5.
Eldredge-Hindy H, Ohri N, Anne PR, et al. Yttrium-90 microsphere brachytherapy for liver metastases from uveal melanoma: clinical outcomes and the predictive value of fluorodeoxyglucose positron emission tomography. Am J Clin Oncol. 2014.
Alexander HR, Libutti SK, Bartlett DL, Puhlmann M, Fraker DL, Bachenheimer LC. A phase I-II study of isolated hepatic perfusion using melphalan with or without tumor necrosis factor for patients with ocular melanoma metastatic to liver. Clin Cancer Res. 2000;6:3062–70.
Alexander HR Jr, Libutti SK, Pingpank JF, et al. Hyperthermic isolated hepatic perfusion using melphalan for patients with ocular melanoma metastatic to liver. Clin Cancer Res. 2003;9:6343–9.
Noter SL, Rothbarth J, Pijl ME, et al. Isolated hepatic perfusion with high-dose melphalan for the treatment of uveal melanoma metastases confined to the liver. Melanoma Res. 2004;14:67–72.
Pingpank JF, Libutti SK, Chang R, et al. Phase I study of hepatic arterial melphalan infusion and hepatic venous hemofiltration using percutaneously placed catheters in patients with unresectable hepatic malignancies. J Clin Oncol. 2005;23:3465–74.
Rizell M, Mattson J, Cahlin C, Hafstrom L, Lindner P, Olausson M. Isolated hepatic perfusion for liver metastases of malignant melanoma. Melanoma Res. 2008;18:120–6.
Verhoef C, de Wilt JH, Brunstein F, et al. Isolated hypoxic hepatic perfusion with retrograde outflow in patients with irresectable liver metastases; a new simplified technique in isolated hepatic perfusion. Ann Surg Oncol. 2008;15:1367–74.
van Iersel LB, Hoekman EJ, Gelderblom H, et al. Isolated hepatic perfusion with 200-mg melphalan for advanced noncolorectal liver metastases. Ann Surg Oncol. 2008;15:1891–8.
van Etten B, de Wilt JH, Brunstein F, Eggermont AM, Verhoef C. Isolated hypoxic hepatic perfusion with melphalan in patients with irresectable ocular melanoma metastases. Eur J Surg Oncol. 2009;35:539–45.
Ben-Shabat I, Hansson C, Sternby Eilard M, et al. Isolated hepatic perfusion as a treatment for liver metastases of uveal melanoma. J Vis Exp. 2015;95:52490.
Ku Y, Saitoh M, Nishiyama H, et al. Extracorporeal adriamycin-removal following hepatic artery infusion: use of direct hemoperfusion combined with veno-venous bypass (in Japanese). Nihon Geka Gakkai Zasshi. 1989;90:1758–64.
Curley SA, Byrd DR, Newman RA, et al. Hepatic arterial infusion chemotherapy with complete hepatic venous isolation and extracorporeal chemofiltration: a feasibility study of a novel system. Anticancer Drugs. 1991;2:175–83.
Beheshti MV, Denny DF Jr, Glickman MG, et al. Percutaneous isolated liver perfusion for treatment of hepatic malignancy: preliminary report. J Vasc Interv Radiol. 1992;3:453–8.
Ravikumar TS, Pizzorno G, Bodden W, et al. Percutaneous hepatic vein isolation and high-dose hepatic arterial infusion chemotherapy for unresectable liver tumors. J Clin Oncol. 1994;12:2723–36.
Curley SA, Newman RA, Dougherty TB, et al. Complete hepatic venous isolation and extracorporeal chemofiltration as treatment for human hepatocellular carcinoma: a phase I study. Ann Surg Oncol. 1994;1:389–99.
Pingpank JF, Hughes MS, Alexander HR, Faries MB, Zager JS, Royal R, Whitman ED, Nutting CW, Siskin GP, Agarwala SS. A phase III random assignment trial comparing percutaneous hepatic perfusion with melphalan (PHP-mel) to standard of care for patients with hepatic metastases from metastatic ocular or cutaneous melanoma. J Clin Oncol. 2010;28(18 Suppl):LBA8512.
Carvajal RD, Schwartz GK, Mann H, Smith I, Nathan PD. Study design and rationale for a randomised, placebo-controlled, double-blind study to assess the efficacy of selumetinib (AZD6244; ARRY-142886) in combination with dacarbazine in patients with metastatic uveal melanoma (SUMIT). BMC Cancer. 2015;15:467.
Olofsson R, Lindberg E, Karlsson-Parra A, Lindner P, Mattsson J, Andersson B. Melan-A-specific CD8 + T lymphocytes after hyperthermic isolated limb perfusion: a pilot study in patients with in-transit metastases of malignant melanoma. Int J Hyperthermia. 2013;29:234–8.
Dudek-Peric AM, Ferreira GB, Muchowicz A, et al. Antitumor immunity triggered by melphalan is potentiated by melanoma cell surface-associated calreticulin. Cancer Res. 2015;75:1603–14.
Olofsson R, Cahlin C, All-Ericsson C, et al. Isolated hepatic perfusion for ocular melanoma metastasis: registry data suggests a survival benefit. Ann Surg Oncol. 2014;21:466–72.
Olofsson R, Ny L, Eilard MS, et al. Isolated hepatic perfusion as a treatment for uveal melanoma liver metastases (the SCANDIUM trial): study protocol for a randomized controlled trial. Trials. 2014;15:317.
Acknowledgments
This study was supported by grants from the Signe and Olof Wallenius Foundation, The Göteborg Medical Society, Assar Gabrielsson Foundation, and the Wilhelm and Marina Lundgren’s Foundation.
Conflict of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben-Shabat, I., Belgrano, V., Ny, L. et al. Long-Term Follow-Up Evaluation of 68 Patients with Uveal Melanoma Liver Metastases Treated with Isolated Hepatic Perfusion. Ann Surg Oncol 23, 1327–1334 (2016). https://doi.org/10.1245/s10434-015-4982-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4982-5