Abstract
Background
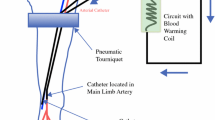
Despite advances in cross-sectional imaging, chemotherapeutic dosing for isolated limb infusion (ILI) in melanoma is currently calculated through cumbersome and potentially imprecise manual measurements. The primary objective of this study was to examine the feasibility of using computed tomography (CT) to calculate limb volume, its concordance with manual measurement, and its ability to predict clinical response and toxicity in patients undergoing ILI.
Methods
A retrospective analysis of all patients undergoing lower extremity ILI at Duke University Medical Center between 2003 and 2014 was performed. Data pertaining to manually measured limb volume, chemotherapeutic dosing, and patient outcome was obtained. CT-based measurements of limb volume were performed in all patients for whom imaging was available and subsequently compared with manually measured values.
Results
CT data were sufficient for measurement in 73 patients. The mean measurement time was 4.61 ± 2.13 min. Although average CT-based measurements were 1.20 L higher in the case of lower limbs, they correlated well with those obtained manually (r 2 = 0.90). Unlike manual measurement, patients with complete responses to chemotherapy had smaller limb volumes than those with disease progression as measured by CT (9.3 vs. 10.7 L; p = .038). Patients suffering grade 3 and 4 toxicities also had statistically lower limb volumes as measured by CT than those who did not (p < .05).
Conclusions
CT-based limb volume measurement is feasible for chemotherapy dosing in patients undergoing ILI for melanoma and has predictive value with respect to clinical response and toxicity.




Similar content being viewed by others
References
Balch CM. Cutaneous melanoma: a review of clinical management. Tex Med. 1987;83:70–8.
Lens MB, Dawes M. Isolated limb perfusion with melphalan in the treatment of malignant melanoma of the extremities: a systematic review of randomised controlled trials. Lancet Oncol. 2003;4:359–64.
Pawlik TM, Ross MI, Johnson MM, et al. Predictors and natural history of in-transit melanoma after sentinel lymphadenectomy. Ann Surg Oncol. 2005;12:587–96.
Coit D, Wallack M, Balch C. Society of Surgical Oncology practice guidelines. Melanoma surgical practice guidelines. Oncology. 1997;11:1317–23.
Beasley GM, Petersen RP, Yoo J, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;15:2195–205.
Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208:706–15; discussion 715–7
Santillan AA, Delman KA, Beasley GM, et al. Predictive factors of regional toxicity and serum creatine phosphokinase levels after isolated limb infusion for melanoma: a multi-institutional analysis. Ann Surg Oncol. 2009;16:2570–8.
McMahon N, Cheng TY, Beasley GM, et al. Optimizing melphalan pharmacokinetics in regional melanoma therapy: does correcting for ideal body weight alter regional response or toxicity? Ann Surg Oncol. 2009;16:953–61.
Wieberdink J, Benckhuysen C, Braat RP, van Slooten EA, Olthuis GA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18:905–10.
van Os J, Schraffordt Koops H, Oldhoff J. Dosimetry of cytostatics in hyperthermic regional isolated perfusion. Cancer. 1985;55:698–701.
Byrne DS, McKay AJ, Blackie R, MacKie RM. A comparison of dosimetric methods in isolated limb perfusion with melphalan for malignant melanoma of the lower extremity. Eur J Cancer. 1996;32A:2082–7.
Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Sem Radiat Oncol. 2003;13:176–81.
Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014. doi:10.1093/jnci/dju244
Geraghty EM, Boone JM, McGahan JP, Jain K. Normal organ volume assessment from abdominal CT. Abdom Imaging. 2004;29:482–90.
Gao L, Heath DG, Kuszyk BS, Fishman EK. Automatic liver segmentation technique for three-dimensional visualization of CT data. Radiology. 1996;201:359–64.
Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–16.
Rosset A, Spadola L, Pysher L, Ratib O. Informatics in radiology (infoRAD): navigating the fifth dimension: innovative interface for multidimensional multimodality image navigation. Radiographics. 2006;26:299–308.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Mohr P, Eggermont AM, Hauschild A, Buzaid A. Staging of cutaneous melanoma. Ann Oncol. 2009;20 Suppl 6:vi14–21.
Reinhardt MJ, Joe AY, Jaeger U, et al. Diagnostic performance of whole body dual modality 18F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: experience with 250 consecutive patients. J Clin Oncol. 2006;24:1178–87.
Holder WD, Jr., White RL, Jr., Zuger JH, Easton EJ, Jr., Greene FL. Effectiveness of positron emission tomography for the detection of melanoma metastases. Ann Surg. 1998;227:764–9; discussion 769–71
Paquet P, Hustinx R, Rigo P, Pierard GE. Malignant melanoma staging using whole-body positron emission tomography. Melanoma Res. 1998;8:59–62.
Rinne D, Baum RP, Hor G, Kaufmann R. Primary staging and follow-up of high risk melanoma patients with whole-body 18F-fluorodeoxyglucose positron emission tomography: results of a prospective study of 100 patients. Cancer. 1998;82:1664–71.
Steinert HC, Huch Boni RA, Buck A, et al. Malignant melanoma: staging with whole-body positron emission tomography and 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1995;195:705–9.
Swetter SM, Carroll LA, Johnson DL, Segall GM. Positron emission tomography is superior to computed tomography for metastatic detection in melanoma patients. Ann Surg Oncol. 2002;9:646–53.
Tyler DS, Onaitis M, Kherani A, et al. Positron emission tomography scanning in malignant melanoma. Cancer. 2000;89:1019–25.
Clauser CE, McConville JT, Young JW. Weight, volume, and center of mass of segments of the human body. J Occupational Env Med. 1971;13:270.
Kroon HM, Moncrieff M, Kam PC, Thompson JF. Factors predictive of acute regional toxicity after isolated limb infusion with melphalan and actinomycin D in melanoma patients. Ann Surg Oncol. 2009;16:1184–92.
Linder P, Doubrovsky A, Kam PC, Thompson JF. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002;9:127–36.
Disclosure
The authors of this manuscript have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brys, A.K., Bhatti, L., Bashir, M.R. et al. Computed Tomography-Based Limb Volume Measurements for Isolated Limb Infusion in Melanoma. Ann Surg Oncol 23, 1090–1095 (2016). https://doi.org/10.1245/s10434-015-4972-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4972-7