Abstract
Background
Perioperative epirubicin, cisplatin, and capecitabine (ECC) chemotherapy was evaluated in patients who underwent esophageal resection for adenocarcinoma of the esophagus or gastroesophageal junction (GEJ).
Methods
A cohort of 93 consecutive patients was analyzed. The median follow-up period was 60 months. Source data verification of adverse events was performed by two independent observers.
Results
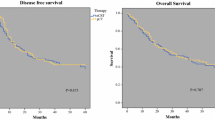
All three planned preoperative chemotherapy cycles were administered to 65 patients (69.9 %). Only 27 % of the patients completed both pre- and postoperative chemotherapy. The reasons for not receiving postoperative adjuvant chemotherapy could be separated in two main problems: toxicity of the preoperative chemotherapy and postoperative problems involving difficulty in recovery and postoperative complications. Finally, 25 patients (27 %), completed three preoperative and three postoperative cycles. Grades 3 and 4 nonhematologic adverse events of preoperative chemotherapy mainly consisted of thromboembolic events (16.2 %) and cardiac complications (7.5 %). A history of cardiac and vascular disease was independently associated with discontinuation of preoperative chemotherapy and the occurrence of grade 3 or higher adverse events. Surgery was performed for 94 % of all the patients who started with ECC chemotherapy. A radical resection (R0) was achieved in 93 % of the patients. A complete pathologic response was observed in 8 % of the patients. During a median follow-up period of 60 months, the median disease-free survival time was 28 months, and the median overall survival time was 36 months. The 3-year overall survival rate was 50 %, and the 5-year overall survival rate was 42 %.
Conclusion
For patients with adenocarcinoma of the esophagus or GEJ, six cycles of ECC-based perioperative chemotherapy is associated with a relatively high number of adverse events. Although this toxicity did not affect the esophageal resectability rate, this regimen should be used with caution in this patient population.


Similar content being viewed by others
References
Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Eng J Med. 2002;347:1662–9.
Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg. 2007;246:992–1000.
Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–7.
Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979–84.
Urba SG, Orringer MB, Turrisi A, et al.: Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–13.
Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–68.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46.
Starling N, Okines A, Cunningham D, et al. A phase II trial of preoperative chemotherapy with epirubicin, cisplatin, and capecitabine for patients with localised gastro-oesophageal junctional adenocarcinoma. Br J Cancer. 2009;100:1725–30.
Verhage RJ, van der Horst S, van der Sluis PC, Lolkema MP, van Hillegersberg R. Risk of thromboembolic events after perioperative chemotherapy versus surgery alone for esophageal adenocarcinoma. Ann Surg Oncol. 2012;19:684–92.
Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Bethesda:National Cancer Institute; 2006.
Sobin LH, Gospodarowicz MK, Wittekind CH, eds. TNM classification of malignant tumors. 7th ed. Wiley-Blackwell, Oxford; 2009.
Verhage RJ, Zandvoort HJ, ten Kate FJ, van Hillegersberg R. How to define a positive circumferential resection margin in T3 adenocarcinoma of the esophagus. Am J Surg Pathol. 2011;35:919–26.
Schnetzler B, Popova N, Collao Lamb C, et al. (2001) Coronary spasm induced by capecitabine. Ann Oncol. 12:723–5.
Falanga A. The incidence and risk of venous thromboembolism associated with cancer and nonsurgical cancer treatment. Cancer Invest. 2009;27:105–15.
Van Custem E, Hoff PM, Blum JL et al. Incidence of cardiotoxicity with oral fluoropyrimidine capecitabine is typical of that reported with 5-fluorouracil. Ann Oncol. 2002;13:484–5.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Brewster DH, Fraser LA, McKinney PA, Black RJ. Socioeconomic status and risk of adenocarcinoma of the oesophagus and cancer of the gastric cardia in Scotland. Br J Cancer. 2000;83:387–90.
Vicari JJ, Peek RM, Falk GW, et al. The seroprevalence of cagA-positive Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology. 1998;115:50–7.
Chow WH, Blaser MJ, Blot WJ, et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998;58:588–90.
Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37.
Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, Langer P, Engenhart-Cabillic R, Bitzer M, Königsrainer A, Budach W, Wilke H. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851–6.
Burmeister BH, Thomas JM, Burmeister EA, Walpole ET, Harvey JA, Thomson DB, Barbour AP, Gotley DC, Smithers BM. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer. 2011;47:354–60.
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A, CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
van Hagen P, Wijnhoven BP, Nafteux P, Moons J, Haustermans K, De Hertogh G, van Lanschot JJ, Lerut T. Recurrence pattern in patients with a pathologically complete response after neoadjuvant chemoradiotherapy and surgery for oesophageal cancer. Br J Surg. 2013;100:267–73.
Conflict of interest
P.C. van der Sluis, I. Ubink, S. van der Horst, J.J. Boonstra, E.E. Voest, J.P. Ruurda, I.H.M. Borel Rinkes, M.J. Wiezer, M.E.I. Schipper, P.D. Siersema, M. Los, M.P. Lolkema, R. van Hillegersberg declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
van der Sluis, P.C., Ubink, I., van der Horst, S. et al. Safety, Efficacy, and Long-Term Follow-Up Evaluation of Perioperative Epirubicin, Cisplatin, and Capecitabine Chemotherapy in Esophageal Resection for Adenocarcinoma. Ann Surg Oncol 22, 1555–1563 (2015). https://doi.org/10.1245/s10434-014-4120-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4120-9