Abstract
Background
The purpose of this study was to determine if gene signatures are informative in colon cancer (CC) when National Quality Standards (NQS) are adhered to. Several studies have demonstrated the prognostic potential of gene signatures in primary CC. This has never been evaluated prospectively with adherence to NQS.
Methods
This was a prospective, international, multicenter trial. Eligibility criteria were: no distant metastasis, ≥12 lymph nodes (LNs), and no adjuvant chemotherapy for LN-negative CC. RNA from frozen tumor samples was considered reliable if RNA Integrity Number >9. Using an Agilent whole human genome array, 44,000 genes were analyzed in primary tumors for differential gene expression (DGE). ANOVA applied at 2-fold expression level was performed in at least 8 experiments to obtain the DGEs.
Results
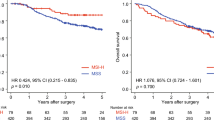
Molecular analysis was completed in 113 of 128 patients. With median follow-up of 27 months, 11.5 % recurred within 3 years after surgery. Significant DGE was identified in recurrent tumors reflected by upregulation (UR) in cellular proliferation and by downregulation (DR) in prodifferentiating panel of 9 genes, independent of T or N classification. By multivariate analysis 3-year disease-free survival was 12.5 % in the UR/DR group versus 93.4 % in the non-UR/DR group (p < .0001; HR = 24.2; 95 % CI 4.8–120.4).
Conclusions
This is the first prospective trial to evaluate gene signatures in CC with adherence to a 12-node minimum quality standard. Certain molecular pathways may be prognostically relevant if both surgery and pathology are standardized, regardless of T or N classification. Careful consideration should be made to include surgical quality measures when planning clinical trials to evaluate the true effect of molecular markers in CC.



Similar content being viewed by others
References
Siegel R, Naishadham D, and Jemal A, Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
Kim SH, Ha TK, and Kwon SJ, Evaluation of the 7th AJCC TNM staging system in point of lymph node classification. J Gastric Cancer. 2011;11:94–100.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual, Colon and rectum. 7th ed. New York: Springer; 2010. pp. 143–64.
Cohen AM, Tremiterra S, Candela F, Thaler HT, Sigurdson ER, Prognosis of node-positive colon cancer. Cancer. 1991;67:1859–61.
Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–73.
Chen SL, Bilchik AJ, More extensive nodal dissection improves survival for stages I to III of colon cancer: a population-based study. Ann Surg. 2006;244:602–10.
Chun P, Wainberg ZA, Adjuvant chemotherapy for stage II colon cancer: the role of molecular markers in choosing therapy. Gastrointest Cancer Res. 2009;3:191–6.
Prosnitz RG, Patwardhan MB, Samsa GP, Mantyh CR, Fisher DA, McCrory DC, et al. Quality measures for the use of adjuvant chemotherapy and radiation therapy in patients with colorectal cancer: a systematic review. Cancer. 2006;107:2352–60.
Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ, Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–9.
Labianca R, Merelli B, Screening and diagnosis for colorectal cancer: present and future. Tumori. 2010;96:889–901.
Hari DM, Leung AM, Lee JH, Sim MS, Vuong B, Chiu CG, et al. AJCC Cancer Staging Manual 7th edition criteria for colon cancer: do the complex modifications improve prognostic assessment? J Am Coll Surg. 2013;217:181–90.
Hari DM, Bilchik AJ. Clinical decision-making and implementation challenges with the AJCC VII staging system for colorectal carcinoma. J Surg Oncol. 2012;105:221–2.
Short SS, Stojadinovic A, Nissan A, Wainberg Z, Dhall D, Yao K, et al. Adjuvant treatment of early colon cancer with micrometastases: results of a national survey. J Surg Oncol. 2012;106:119–22.
Chou JF, Row D, Gonen M, Liu YH, Schrag D, Weiser MR. Clinical and pathologic factors that predict lymph node yield from surgical specimens in colorectal cancer: a population-based study. Cancer. 2010;116:2560–70.
Stojadinovic A, Nissan A, Wainberg Z, Shen P, McCarter M, Protic M, et al. Time-dependent trends in lymph node yield and impact on adjuvant therapy decisions in colon cancer surgery: an international multi-institutional study. Ann Surg Oncol. 2012;19:4178–85.
Belt EJ, te Velde EA, Krijgsman O, Brosens RP, Tijssen M, van Essen HF, et al. High lymph node yield is related to microsatellite instability in colon cancer. Ann Surg Oncol. 2012;19:1222–30.
Eveno C, Nemeth J, Soliman H, Praz F, de The H, Valleur P, et al. Association between a high number of isolated lymph nodes in T1 to T4 N0M0 colorectal cancer and the microsatellite instability phenotype. Arch Surg. 2010;145:12–7.
Soreide K, Nedrebo BS, Soreide JA, Slewa A, Korner H, Lymph node harvest in colon cancer: influence of microsatellite instability and proximal tumor location. World J Surg. 2009;33:2695–703.
Nissan A, Protic M, Bilchik AJ, Howard RS, Peoples GE, Stojadinovic A. United States Military Cancer Institute Clinical Trials Group (USMCI GI-01) randomized controlled trial comparing targeted nodal assessment and ultrastaging with standard pathological evaluation for colon cancer. Ann Surg. 2012;256:412–27.
Mescoli C, Albertoni L, Pucciarelli S, Giacomelli L, Russo VM, Fassan M, et al. Isolated tumor cells in regional lymph nodes as relapse predictors in stage I and II colorectal cancer. J Clin Oncol. 2012;30:965–71.
Sloothaak DA, Sahami S, van der Zaag-Loonen HJ, van der Zaag ES, Tanis PJ, Bemelman WA, et al. The prognostic value of micrometastases and isolated tumour cells in histologically negative lymph nodes of patients with colorectal cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2014;40:263–9.
Venook AP, Niedzwiecki D, Lopatin M, Lee M, Friedman PN, Frankel W, et al. Validation of a 12-gene colon cancer recurrence score (RS) in patients (pts) with stage II colon cancer (CC) from CALGB 9581. J Clin Oncol. 2011;29:suppl; abstr 3518.
Kerr D, Gray R, Quirke P, Watson D, Yothers G, Lavery IC, et al. A quantitative multigene RT-PCR assay for prediction of recurrence in stage II colon cancer: Selection of the genes in four large studies and results of the independent, prospectively designed QUASAR validation study. J Clin Oncol. 2009;27:suppl; abstr 4000.
Yothers G, O’Connell MJ, Lee M, Lopatin M, Clark-Langone KM, Millward C, et al. Validation of the 12-gene colon cancer recurrence score in NSABP C-07 as a predictor of recurrence in patients with stage II and III colon cancer treated with fluorouracil and leucovorin (FU/LV) and FU/LV plus oxaliplatin. J Clin Oncol. 2013;31:4512–9.
O’Connell MJ, Lee M, Lopatin M, Yothers G, Clark-Langone K, Millward C, et al. Validation of the 12-gene colon cancer Recurrence Score (RS) in NSABP C-07 as a predictor of recurrence in stage II and III colon cancer patients treated with 5FU/LV (FU) and 5FU/LV + oxaliplatin (FU + Ox). J Clin Oncol. 2012;30;suppl; abstr 3512.
Maak M, Simon I, Nitsche U, Roepman P, Snel M, Glas AM, et al. Independent validation of a prognostic genomic signature (ColoPrint) for patients with stage II colon cancer. Ann Surg. 2013;257:1053–8.
Tabernero J, Moreno V, Rosenberg R, Nitsche U, Bachleitner-Hofmann T, Lanza G, et al. Clinical and technical validation of a genomic classifier (ColoPrint) for predicting outcome of patients with stage II colon cancer. J Clin Oncol. 2012;30;suppl; abstr 384.
Salazar R, Roepman P, Capella G, Moreno V, Simon I, Dreezen C, et al. Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. J Clin Oncol. 2011;29:17–24.
Acknowledgment
Thanks to Tiffany Felix, Dr. Alix Norris, the Henry M. Jackson Foundation for the Advancement of Military Medicine, and the Bon Secours Virginia Foundation.
Disclosure
The authors have no financial or commercial interest related to this research. The financial and material support from this project originated from NIH R01 Grant (5RO1CA090848), United States Military Cancer Institute (USMCI), and the California Oncology Research Institute (CORI). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bilchik, A.J., Wainberg, Z.A., Nissan, A. et al. Value of Primary Tumor Gene Signatures in Colon Cancer When National Quality Standards are Adhered to: Preliminary Results of an International Prospective Multicenter Trial. Ann Surg Oncol 22, 535–542 (2015). https://doi.org/10.1245/s10434-014-4013-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4013-y