Abstract
Background
Intraoperative cell salvage (IOCS) has been used in musculoskeletal surgery extensively. However, it has never found its place in musculoskeletal oncologic surgery. We have conducted the first-ever study to evaluate the feasibility of IOCS in combination with a leucocyte-depletion filter (LDF) in metastatic spine tumor surgery. This was to pave the path for use of IOCS-LDF in musculoskeletal oncologic surgery.
Methods
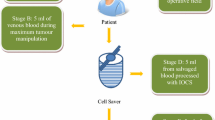
Patients with a known primary epithelial tumor, who were offered surgery for metastatic spinal disease, were recruited. Blood samples were collected at three different stages during the surgery: from the operative field before IOCS processing, after IOCS processing, and after IOCS-LDF processing. Three separate samples (5 mL each) were taken at each stage. Samples were examined using immunohistochemical monoclonal antibodies to identify tumor cells of epithelial origin.
Results
Of 30 patients in the study, 6 were excluded for not fulfilling the inclusion criteria, leaving 24 patients. Malignant tumor cells were detected in the samples from the operative field before IOCS processing in eight patients and in the samples from the transfusion bag after IOCS processing in three patients. No viable malignant cell was detectable in any of the blood samples after passage through both IOCS and LDF.
Conclusions
The findings support the notion that the IOCS-LDF combination works effectively in eliminating tumor cells from salvaged blood, so this technique can be applied successfully in spine tumor surgery. This concept can then further be extended to whole musculoskeletal tumor surgery and other oncologic surgeries with further appropriate clinical studies.


Similar content being viewed by others
References
Jacobs WB, Perrin RG. Evaluation and treatment of spinal metastases: an overview. Neurosurg Focus. 2001;11:e10.
Bilsky MH, Fraser JF. Complication avoidance in vertebral column spine tumors. Neurosurg Clin N Am. 2006;17:317–329, vii.
Chen Y, Tai BC, Nayak D, et al. Blood loss in spinal tumour surgery and surgery for metastatic spinal disease: a meta-analysis. Bone Joint J. 2013;95-B:683–8.
Kawai A, Kadota H, Yamaguchi U, Morimoto Y, Ozaki T, Beppu Y. Blood loss and transfusion associated with musculoskeletal tumor surgery. J Surg Oncol. 2005;92:52–8.
Miller GV, Ramsden CW, Primrose JN. Autologous transfusion: an alternative to transfusion with banked blood during surgery for cancer. Br J Surg. 1991;78:713–5.
Cata JP, Gottumukkala V. Blood loss and massive transfusion in patients undergoing major oncological surgery: what do we know? ISRN Anesthesiol. Vol. 2012, Article ID 918938.
Ashworth A, Klein AA. Cell salvage as part of a blood conservation strategy in anaesthesia. Br J Anaesth. 2010;105:401–16.
Blajchman MA, Bordin JO. The tumor growth-promoting effect of allogeneic blood transfusions. Immunol Invest. 1995;24:311–7.
Blumberg N. Allogeneic transfusion and infection: economic and clinical implications. Semin Hematol. 1997;34(3 Suppl. 2):34–40.
Kumar N, Chen Y, Nath C, Liu EH. What is the role of autologous blood transfusion in major spine surgery? Am J Orthop (Belle Mead NJ). 2012;41:E89–95.
Cavallieri S, Riou B, Roche S, Ducart A, Roy-Camille R, Viars P. Intraoperative autologous transfusion in emergency surgery for spine trauma. J Trauma. 1994;36:639–43.
Munoz M, Garcia-Vallejo JJ, Ruiz MD, Romero R, Olalla E, Sebastian C. Transfusion of post-operative shed blood: laboratory characteristics and clinical utility. Eur Spine J. 2004;13(Suppl. 1):S107–13.
Blundell J. Some account of a case of obstinate vomiting, in which an attempt was made to prolong life by the injection of blood into the veins. Med Chir Trans. 1819;10(Pt. 2):296–311.
Savvidou C, Chatziioannou SN, Pilichou A, Pneumaticos SG. Efficacy and cost-effectiveness of cell saving blood autotransfusion in adult lumbar fusion. Transfus Med. 2009;19:202–6.
Flynn JC, Metzger CR, Csencsitz TA. Intraoperative autotransfusion (IAT) in spinal surgery. Spine (Phila Pa 1976). 1982;7:432–5.
McKenna SG. Autologous blood transfusions: Council on Scientific Affairs. JAMA. 1986;256:2378–80.
Catling S, Williams S, Freites O, Rees M, Davies C, Hopkins L. Use of a leucocyte filter to remove tumour cells from intra-operative cell salvage blood. Anaesthesia. 2008;63:1332–8.
Waters JH, Yazer M, Chen YF, Kloke J. Blood salvage and cancer surgery: a meta-analysis of available studies. Transfusion. 2012;52:2167–73.
Yaw PB, Sentany M, Link WJ, Wahle WM, Glover JL. Tumor cells carried through autotransfusion: contraindication to intraoperative blood recovery? JAMA. 1975;231:490–1.
Gwak MS, Lee KW, Kim SY, et al. Can a leukocyte depletion filter (LDF) reduce the risk of reintroduction of hepatocellular carcinoma cells? Liver Transpl. 2005;11:331–5.
Liang TB, Li DL, Liang L, et al. Intraoperative blood salvage during liver transplantation in patients with hepatocellular carcinoma: efficiency of leukocyte depletion filters in the removal of tumor cells. Transplantation. 2008;85:863–9.
Kumar N, Chen Y, Zaw AS, et al. Use of intraoperative cell-salvage for autologous blood transfusions in metastatic spine tumour surgery: a systematic review. Lancet Oncol. 2014;15:e33–41.
Connor JP, Morris PC, Alagoz T, Anderson B, Bottles K, Buller RE. Intraoperative autologous blood collection and autotransfusion in the surgical management of early cancers of the uterine cervix. Obstet Gynecol. 1995;86:373–8.
Mirhashemi R, Averette HE, Deepika K, et al. The impact of intraoperative autologous blood transfusion during type III radical hysterectomy for early-stage cervical cancer. Am J Obstet Gynecol. 1999;181:1310–5; discussion 1315–6.
Bower MR, Ellis SF, Scoggins CR, McMasters KM, Martin RC. Phase II comparison study of intraoperative autotransfusion for major oncologic procedures. Ann Surg Oncol. 2011;18:166–73.
Fujimoto J, Okamoto E, Yamanaka N, et al. Efficacy of autotransfusion in hepatectomy for hepatocellular carcinoma. Arch Surg. 1993;128:1065–9.
Kim JM, Kim GS, Joh JW, et al. Long-term results for living donor liver transplant recipients with hepatocellular carcinoma using intraoperative blood salvage with leukocyte depletion filter. Transpl Int. 2012;26:84–9.
Martin RC, Wellhausen SR, Moehle DA, Martin AW, McMasters KM. Evaluation of intraoperative autotransfusion filtration for hepatectomy and pancreatectomy. Ann Surg Oncol. 2005;12:1017–24.
Muscari F, Suc B, Vigouroux D, et al. Blood salvage autotransfusion during transplantation for hepatocarcinoma: does it increase the risk of neoplastic recurrence? Transpl Int. 2005;18:1236–9.
Perseghin P, Vigano M, Rocco G, Della Pona C, Buscemi A, Rizzi A. Effectiveness of leukocyte filters in reducing tumor cell contamination after intraoperative blood salvage in lung cancer patients. Vox Sang. 1997;72:221–4.
Davis M, Sofer M, Gomez-Marin O, Bruck D, Soloway MS. The use of cell salvage during radical retropubic prostatectomy: does it influence cancer recurrence? BJU Int. 2003;91:474–6.
Gorin MA, Eldefrawy A, Manoharan M, Soloway MS. Oncologic outcomes following radical prostatectomy with intraoperative cell salvage. World J Urol. 2012;30:379–83.
Klimberg I, Sirois R, Wajsman Z, Baker J. Intraoperative autotransfusion in urologic oncology. Arch Surg. 1986;121:1326–9.
MacIvor D, Nelson J, Triulzi D. Impact of intraoperative red blood cell salvage on transfusion requirements and outcomes in radical prostatectomy. Transfusion. 2009;49:1431–4.
Nieder AM, Carmack AJ, Sved PD, Kim SS, Manoharan M, Soloway MS. Intraoperative cell salvage during radical prostatectomy is not associated with greater biochemical recurrence rate. Urology. 2005;65:730–4.
Nieder AM, Manoharan M, Yang Y, Soloway MS. Intraoperative cell salvage during radical cystectomy does not affect long-term survival. Urology. 2007;69:881–4.
Poli M, Camargo A, Villa L, Moura R, Colella R, Deheinzelin D. Intraoperative autologous blood recovery in prostate cancer surgery: in vivo validation using a tumour marker. Vox Sang. 2008;95:308–12.
Park KI, Kojima O, Tomoyoshi T. Intra-operative autotransfusion in radical cystectomy. Br J Urol. 1997;79:717–21.
Gokaslan ZL, York JE, Walsh GL, et al. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg. 1998;89:599–609.
Hage WD, Aboulafia AJ, Aboulafia DM. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop Clin North Am. 2000;31:515–28, vii.
Amin R. Intramedullary spinal metastasis from carcinoma of the cervix. Br J Radiol. 1999;72:89–91.
Colletti PM, Siegel HJ, Woo MY, Young HY, Terk MR. The impact on treatment planning of MRI of the spine in patients suspected of vertebral metastasis: an efficacy study. Comput Med Imaging Graph. 1996;20:159–62.
Listrom MB, Dalton LW. Comparison of keratin monoclonal antibodies MAK-6, AE1:AE3, and CAM-5.2. Am J Clin Pathol. 1987;88:297–301.
Tseng SCG, Jarvinen MJ, Nelson WG, Huang J-W, Woodcock-Mitchell J, Sun T–T. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982;30:361–72.
Moll R. Cytokeratins as markers of differentiation in the diagnosis of epithelial tumors. Subcell Biochem. 1998;31:205–62.
Foltys D, Zimmermann T, Heise M, et al. Liver transplantation for hepatocellular carcinoma: is there a risk of recurrence caused by intraoperative blood salvage autotransfusion? Eur Surg Res. 2011;47:182–7.
Acknowledgment
The authors thank Ms. Ee Yong Foo from the Department of Pathology for technical assistance in preparation of the cell blocks used in this study; A/Prof Gabriel Liu, A/Prof Joseph Thambiah, A/Prof Gamaliel Tan YH and Dr Tony Setiobudi for contributing their cases to the study; Anaesthesia Unit (AU) Nurses at the NUHS Singapore for supporting the study by managing the IOCS machine & the LDF system. This research was supported by the National Medical Research Council, Singapore. We were awarded the grant in May 2011 to conduct a prospective study (grant NMRC/NIG/1055/2011; value of award: SGD 200,000.00; DSRB/IRB reference: 2011/00350).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conference presentation, “Eliminating Tumour Cells from the Cell Saver (CS) in Metastatic Spine Tumour Surgery Using a Leucocyte Depletion Filter (LDF): Dispelling an Old Myth”, N. Kumar, Y. Chen, Q. Ahmed, V. K. M. Lee, A. S. Zaw, R. Goy, H. K. Wong was awarded the Kostuik Innovation Award at the International Society for Advancement of Spine Surgery (ISASS13) Tier 1 Spine meeting in Vancouver, Canada, April 3–5 2013.
Rights and permissions
About this article
Cite this article
Kumar, N., Ahmed, Q., Lee, V.K.M. et al. Can There be a Place for Intraoperative Salvaged Blood in Spine Tumor Surgery?. Ann Surg Oncol 21, 2436–2443 (2014). https://doi.org/10.1245/s10434-014-3569-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-3569-x