Abstract
Background
Surgical resection is the only curative strategy for pancreatic ductal adenocarcinoma (PDAC), but recurrence rates are high even after purported curative resection. First-line treatment with gemcitabine and S-1 (GS) is associated with promising antitumor activity with a high response rate. The aim of this study was to assess the feasibility and efficacy of GS in the neoadjuvant setting.
Methods
In a multi-institutional single-arm phase 2 study, neoadjuvant chemotherapy (NAC) with gemcitabine and S-1, repeated every 21 days, was administered for two cycles (NAC-GS) to patients with resectable and borderline PDAC. The primary end point was the 2-year survival rate. Secondary end points were feasibility, resection rate, pathological effect, recurrence-free survival, and tumor marker status.
Results
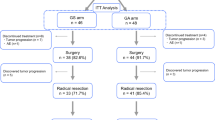
Of 36 patients enrolled, 35 were eligible for this clinical trial conducted between 2008 and 2010. The most common toxicity was neutropenia in response to 90 % of the relative dose intensity. Responses to NAC included radiological tumor shrinkage (69 %) and decreases in CA19-9 levels (89 %). R0 resection was performed for 87 % in resection, and the morbidity rate (40 %) was acceptable. The 2-year survival rate of the total cohort was 45.7 %. Patients who underwent resection without metastases after NAC-GS (n = 27) had an increased median overall survival (34.7 months) compared with those who did not undergo resection (P = 0.0017).
Conclusions
NAC-GS was well tolerated and safe when used in a multi-institutional setting. The R0 resection rate and the 2-year survival rate analysis are encouraging for patients with resectable and borderline PDAC.



Similar content being viewed by others
References
Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57.
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96.
Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–17.
Matsuno S, Egawa S, Fukuyama S, et al. Pancreatic cancer registry in Japan: 20 years of experience. Pancreas. 2004;28:219–30.
Smeenk HG, van Eijck CH, Hop WC, et al. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg. 2007;246:734–40.
Gutt R, Liauw SL, Weichselbaum RR. Adjuvant radiotherapy for resected pancreatic cancer: a lack of benefit or a lack of adequate trials? Nat Clin Pract Gastroenterol Hepatol. 2009;6:38–46.
Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10.
Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–77.
Ueno H, Kosuge T, Matsuyama Y, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101:908–15.
Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs. gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–81.
Katz MH, Fleming JB, Lee JE, Pisters PW. Current status of adjuvant therapy for pancreatic cancer. Oncologist. 2010;15:1205–13.
Reni M. Neoadjuvant treatment for resectable pancreatic cancer: time for phase III testing? World J Gastroenterol. 2010;16:4883–7.
Artinyan A, Anaya DA, McKenzie S, Ellenhorn JD, Kim J. Neoadjuvant therapy is associated with improved survival in resectable pancreatic adenocarcinoma. Cancer. 2011;117:2044–9.
Palmer DH, Stocken DD, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14:2088–96.
Heinrich S, Pestalozzi BC, Schäfer M, Weber A, Bauerfeind P, Knuth A, Clavien PA. Prospective phase II trial of neoadjuvant chemotherapy with gemcitabine and cisplatin for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:2526–31.
Sahora K, Kuehrer I, Eisenhut A, et al. NeoGemOx: gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery. 2011;149:311–20.
Shirasaka T, Shimamato Y, Ohshimo H, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–57.
Okusaka T, Funakoshi A, Furuse J, et al. A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2008;61:615–21.
Morizane C, Okusaka T, Furuse J, et al. A phase II study of S-1 in gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2009;63:313–9.
Ueno H, Okusaka T, Ikeda M, et al. A phase I study of combination chemotherapy with gemcitabine and oral S-1 for advanced pancreatic cancer. Oncology. 2005;69:421–7.
Lee GW, Kim HJ, Ju JH, et al. Phase II trial of S-1 in combination with gemcitabine for chemo-naïve patients with locally advanced or metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2009;64:707–13.
Nakai Y, Isayama H, Sasaki T, et al. A pilot study for combination chemotherapy using gemcitabine and S-1 for advanced pancreatic cancer. Oncology. 2009;77:300–3.
Oh DY, Cha Y, Choi IS, et al. A multicenter phase II study of gemcitabine and S-1 combination chemotherapy in patients with unresectable pancreatic cancer. Cancer Chemother Pharmacol. 2010;65:527–36.
Hirano S, Kondo S, Hara T, et al. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: long-term results. Ann Surg. 2007;246:46–51.
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Padhani AR, Ollivier L. The RECIST (Response Evaluation Criteria in Solid Tumors) criteria: implications for diagnostic radiologists. Br J Radiol. 2001;74:983–6.
American Joint Committee on Cancer. AJCC cancer staging manual. 6th ed. Chicago: Springer; 2002.
Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–9.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines). Pancreatic adenocarcinoma. Version 2. 2012 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
Ioka T, Ikeda M, Ohkawa S, et al. Randomized phase III study of gemcitabine plus S-1 (GS) versus S-1 versus gemcitabine (GEM) in unresectable advanced pancreatic cancer (PC) in Japan and Taiwan: GEST study (abstract). J Clin Oncol. 2011;29:4007.
Piperdi M, McDade TP, Shim JK, et al. A neoadjuvant strategy for pancreatic adenocarcinoma increases the likelihood of receiving all components of care: lessons from a single-institution database. HPB (Oxford). 2010;12:204–10.
Le Scodan R, Mornex F, Girard N, et al. Preoperative chemoradiation in potentially resectable pancreatic adenocarcinoma: feasibility, treatment effect evaluation and prognostic factors, analysis of the SFRO-FFCD 9704 trial and literature review. Ann Oncol. 2009;20:1387–96.
Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60.
Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7: e1000267.
VanHouten JP, White RR, Jackson GP. A decision model of therapy for potentially resectable pancreatic cancer. J Surg Res. 2012;174:222–30.
Heinrich S, Pestalozzi B, Lesurtel M, et al. Adjuvant gemcitabine versus neoadjuvant gemcitabine/oxaliplatin plus adjuvant gemcitabine in resectable pancreatic cancer: a randomized multicenter phase III study (NEOPAC study). BMC Cancer. 2011;11:346.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF). Surgery. 2005:138:8–13.
Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007:142:761–8.
Acknowledgment
Supported in part by Grants-in-Aid for Scientific Research 21591766 from the Japan Society for the Promotion of Science. We thank Dr. Tetsuyuki Uchiyama for his contribution to this study.
Disclosure
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Motoi, F., Ishida, K., Fujishima, F. et al. Neoadjuvant Chemotherapy with Gemcitabine and S-1 for Resectable and Borderline Pancreatic Ductal Adenocarcinoma: Results from a Prospective Multi-institutional Phase 2 Trial. Ann Surg Oncol 20, 3794–3801 (2013). https://doi.org/10.1245/s10434-013-3129-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-013-3129-9