Abstract
Background
The objective of this study was to provide a descriptive analysis of registered clinical trials in surgical oncology at ClinicalTrials.gov.
Methods
Data was extracted from ClinicalTrials.gov using the following search engine criteria: “Cancer” as Condition, “Surgery OR Operation OR Resection” as Intervention, and Non-Industry sponsored. The search was limited to Canada and the United States and included trials registered from January 1, 2001 to January 1, 2011.
Results
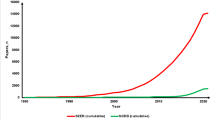
Of 9,961 oncology trials, 1,049 (10.5 %) included any type of surgical intervention. Of these trials, 125 (11.9 %, 1.3 % of all oncology trials) assessed a surgical variable, 773 (73.7 %) assessed adjuvant/neoadjuvant therapies, and 151 (14.4 %) were observational studies. Of the trials assessing adjuvant therapies, systemic treatment (362 trials, 46.8 %) and multimodal therapy (129 trials, 16.7 %) comprised a large focus. Of the 125 trials where surgery was the intervention, 59 trials (47.2 %) focused on surgical techniques or devices, 45 trials (36.0 %) studied invasive diagnostic methods, and 21 trials (16.8 %) evaluated surgery versus no surgery. The majority of the 125 trials were nonrandomized (72, 57.6 %).
Conclusions
The number of registered surgical oncology trials is small in comparison to oncology trials as a whole. Clinical trials specifically designed to assess surgical interventions are vastly outnumbered by trials focusing on adjuvant therapies. Randomized surgical oncology trials account for <1 % of all registered cancer trials. Barriers to the design and implementation of randomized trials in surgical oncology need to be clarified in order to facilitate higher-level evidence in surgical decision-making.


Similar content being viewed by others
References
American Society of Clinical Oncology Mission Statement. http://www.asco.org/ASCOv2/About+ASCO/ASCO+Information/Mission+Statement. Accessed Feb 9 2013.
National Cancer Institute Mission Statement. http://www.cancer.gov/aboutnci/overview/mission. Accessed Feb 9 2013.
Panesar SS, Thakrar R, Athanasiou T, Sheikh A. Comparison of reports of randomized controlled trials and systematic reviews in surgical journals: literature review. J R Soc Med. 2006;99:470–2.
Wente MN, Seiler CM, Uhl W, Büchler MW. Perspectives of evidence-based surgery. Dig Surg. 2003;20:263–9.
Howes N, Chagla L, Thorpe M, McCulloch P. Surgical practice is evidence based. Br J Surg. 1997;84:1220–3.
Ellis J, Mulligan I, Rowe J, Sackett DL. Inpatient general medicine is evidence based. Lancet. 1995;364:407–10.
ClinicalTrials.gov registry. http://www.clinicaltrials.gov/. Accessed Feb 29 2012.
Bailey CS, Fehlings MG, Rampersaud YR, Hall H, Wai EK, Fisher CG, et al. Industry and evidence-based medicine: believable or conflicted? A systematic review of the surgical literature. Can J Surg. 2011;54:321–6.
Okike K, Kocher MS, Mehlman CT, Bhandari M. Conflict of interest in orthopaedic research: an association between findings and fundings in scientific presentations. J Bone Joint Surg. 2007;89:608–13.
Pitak-Arnnop P, Sader R, Rapidis AD, Dhanuthai K, Bauer U, Herve C, et al. Publication bias in oral and maxillofacial surgery journals: an observation on published controlled trials. J Craniomaxillofac Surg. 2010;38:4–10.
Kanaan Z, Galandiuk S, Abby M, Shannon KV, Dajani D, Hicks N, et al. The value of lesser-impact-factor surgical journals as a source of negative and inconclusive outcomes reporting. Ann Surg. 2011;253:619–23.
National Institutes of Health. National Cancer Institute. Common Cancer Types. http://cancer.gov/cancertopics/types/commoncancers. Accessed Mar 2 2012.
McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D, et al. Randomised trials in surgery: problems and possible solutions. BMJ. 2002;324:1448–51.
Sekeres M, Gold JL, Chan A, Lexchin J, Moher D, Van Laethem ML, et al. Poor reporting of scientific leadership information in clinical trial registers. PLoS One. 2008;3:e1610.
International Standard Randomised Controlled Trial Number (ISRCTN) registry. http://www.controlled-trials.com/isrctn/search.html. Accessed Feb 29 2012.
Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The clinicaltrials.gov results database – update and key issues. N Engl J Med. 2011;364:852–60.
metaRegister of Controlled Trials (mRCT). http://www.controlled-trials.com/mrct/. Accessed Feb 29 2012.
Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in clinicaltrials.gov, 2007–2010. JAMA. 2012;307:1838–47.
Zarin DA, Tse T, Ide NC. Trial registration at clinicaltrials.gov between May and October 2005. N Engl J Med. 2005;353:2779–87.
Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in clinicaltrials.gov: a cross-sectional analysis. PLoS Med. 2009;6:e1000144.
Huic M, Marusic M, Marusic A. Completeness and changes in registered data and reporting bias of randomized controlled trials in ICMJE journals after trial registration policy. PLoS One. 2011;6:e25258.
Viergever RF, Ghersi D. The quality of registration of clinical trials. PLoS One. 2011;6:e14701.
World Health Organization. International Clinical Trials Registry Platform. Trial registration data set. http://www.who.int/ictrp/network/trds/en/index.html. Accessed Feb 29, 2012.
Ramsey S, Scoggins J. Practicing on the tip of an information iceberg? Evidence of underpublication of registered clinical trials in oncology. Oncologist. 2008;13:925–9.
Van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–50.
Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–45.
d’Amato TA, Ashrafi AS, Schuchert MJ, Alshehab DS, Seely AJ, Shamji FM, et al. Risk of pneumonectomy after induction therapy for locally advanced non-small cell lung cancer. Ann Thor Surg. 2009;88:1079–85.
Angarita FA, Acuna SA, Torregrosa L, Tawil M, Escallon J, Ruíz Á. Perioperative variables associated with surgical site infection in breast cancer surgery. J Hosp Infect. 2011;79:328–32.
Thornton AD, Ravn P, Winslet M, Chester K. Angiogenesis inhibition with bevacizumab and the surgical management of colorectal cancer. Br J Surg. 2006;93:1456–63.
Wright G, Manser RL, Byrnes G, Hart D, Campbell DA. Surgery for non-small cell lung cancer: systematic review and meta-analysis of randomised controlled trials. Thorax. 2006;61:597–603.
McLeod RS, Wright JG, Solomon MJ, Hu X, Walters BC, Lossing Al. Randomized controlled trials in surgery: issues and problems. Surgery. 1996;119:483–6.
Farrokhyar F, Karanicolas PJ, Thoma A, Simunovic M, Bhandari M, Devereaux PJ, et al. Randomized controlled trials of surgical interventions. Ann Surg. 2010;251:409–16.
Cook JA. The challenges faced in the design, conduct and analysis of surgical randomised controlled trials. Trials. 2009;10:9.
Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT. Statistical assessment of the learning curves of health technologies. Health Technol Assess. 2001;5:1–79.
Young JM, Solomon MJ, Harrison JD, Salkeld G, Butow P. Measuring patient preference and surgeon choice. Surgery. 2008;143:582–8.
Disclosure
This manuscript has not previously been published, nor has it been submitted to any other journal for consideration of publication. This manuscript is intended to analyze registered clinical trials in surgical oncology within clinicaltrials.gov. None of the authors have any financial support, any commercial interest in the subject of the study, or any involvement with an organization with a financial interest in this topic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Menezes, A.S., Barnes, A., Scheer, A.S. et al. Clinical Research in Surgical Oncology: An Analysis of ClinicalTrials.gov. Ann Surg Oncol 20, 3725–3731 (2013). https://doi.org/10.1245/s10434-013-3054-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-013-3054-y