Abstract
Background
The clinical significance of Brachyury expression and its relationship to epithelial–mesenchymal transition in primary lung carcinoma is unclear.
Methods
Expression of Brachyury mRNA was investigated in 104 surgically resected primary lung carcinoma tissues. Immunohistochemical analysis of Brachyury transcription factor, Slug, E-cadherin, IL-8, N-cadherin, and Ki67 was performed in 67 of 104 cases, and their expression was correlated to prognoses and clinicopathological factors.
Results
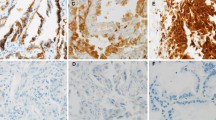
Brachyury mRNA expression in primary lung carcinoma tissues was a significant predictor of poor prognosis for 5-year disease-free survival and overall survival rates and was significantly correlated to vascular invasion, lymphatic permeation, histological grade, pathologic T stage, and pathologic N stage (P < 0.05). Brachyury mRNA expression was significantly inversely correlated to E-cadherin expression (P = 0.0252) and positively correlated to IL-8 protein (P = 0.0241) and to Slug protein (P = 0.0243) in adenocarcinoma tissues.
Conclusions
A positive association between Brachyury and Slug and IL-8, and a negative association with E-cadherin may lead to invasiveness and metastasis in primary lung carcinoma. Brachyury mRNA expression is a significant predictor of poor prognosis in primary lung carcinoma.


Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49.
Zhu CQ, Shih W, Ling CH, Tsao MS. Immunohistochemical markers of prognosis in non–small cell lung cancer: a review and proposal for a multiphase approach to marker evaluation. J Clin Pathol. 2006;59:790–800.
Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–8.
Radisky ES, Radisky DC. Matrix metalloproteinase–induced epithelial–mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:201–12.
Xu J, Rodriguez D, Petitclerc E, et al. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–79.
Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74.
Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial–mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71:5296–306.
Fernando RI, Litzinger M, Trono P, et al. The T-box transcription factor Brachyury promotes epithelial–mesenchymal transition in human tumor cells. J Clin Invest. 2010;120:533–44.
Roselli M, Fernando RI, Guadagni F, et al. Brachyury, a driver of the epithelial–mesenchymal transition, is overexpressed in human lung tumors: an opportunity for novel interventions against lung cancer. Clin Cancer Res. 2012;18:3868–79.
Palena C, Polev DE, Tsang KY, et al. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res. 2007;13:2471–8.
Kilic N, Feldhaus S, Kilic E, et al. Brachyury expression predicts poor prognosis at early stages of colorectal cancer. Eur J Cancer. 2011;47:1080–5.
Krukovskaia LL, Polev DE, Nosova Iu K, et al.[Investigation of transcription factor Brachyury (T) expression in human normal and tumor tissues] Vopr Onkol. 2008;54:739–43.
Park JC, Chae YK, Son CH, et al. Epigenetic silencing of human T (brachyury homologue) gene in non-small-cell lung cancer. Biochem Biophys Res Commun. 2008;365:221–6.
Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–28.
Huber MA, Kraut N, Beug H. Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–58.
Storci G, Sansone P, Trere D, et al. The basal-like breast carcinoma phenotype is regulated by SLUG gene expression. J Pathol. 2008;214:25–37.
Zhang K, Chen D, Jiao X, et al. Slug enhances invasion ability of pancreatic cancer cells through upregulation of matrix metalloproteinase-9 and actin cytoskeleton remodeling. Lab Invest. 2011;91:426–38.
Shih JY, Tsai MF, Chang TH, et al. Transcription repressor slug promotes carcinoma invasion and predicts outcome of patients with lung adenocarcinoma. Clin Cancer Res. 2005;11:8070–8.
Chou TY, Chen WC, Lee AC, et al. Clusterin silencing in human lung adenocarcinoma cells induces a mesenchymal-to-epithelial transition through modulating the ERK/Slug pathway. Cell Signal. 2009;21:704–11.
Cai M, Onoda K, Takao M, et al. Degradation of tenascin-C and activity of matrix metalloproteinase-2 are associated with tumor recurrence in early stage non–small cell lung cancer. Clin Cancer Res. 2002;8:1152–6.
Roussos ET, Condeelis JS, Patsialou A. Chemotaxis in cancer. Nat Rev Cancer. 2011;11:573–87.
Kubo F, Ueno S, Hiwatashi K, et al. Interleukin 8 in human hepatocellular carcinoma correlates with cancer cell invasion of vessels but not with tumor angiogenesis. Ann Surg Oncol. 2005;12:800–7.
Bates RC, DeLeo MJ, 3rd, Mercurio AM. The epithelial–mesenchymal transition of colon carcinoma involves expression of IL-8 and CXCR-1-mediated chemotaxis. Exp Cell Res. 2004;299:315–24.
Kase S, Sugio K, Yamazaki K, et al. Expression of E-cadherin and beta-catenin in human non-small cell lung cancer and the clinical significance. Clin Cancer Res. 2000;6:4789–96.
Masuya D, Huang C, Liu D, et al. The intratumoral expression of vascular endothelial growth factor and interleukin-8 associated with angiogenesis in nonsmall cell lung carcinoma patients. Cancer. 2001;92:2628–38.
Chen JJ, Yao PL, Yuan A, et al. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res. 2003;9:729–37.
Orditura M, De Vita F, Catalano G, et al. Elevated serum levels of interleukin-8 in advanced non–small cell lung cancer patients: relationship with prognosis. J Interferon Cytokine Res. 2002;22:1129–35.
Yuan A, Yang PC, Yu CJ, et al. Interleukin-8 messenger ribonucleic acid expression correlates with tumor progression, tumor angiogenesis, patient survival, and timing of relapse in non-small-cell lung cancer. Am J Respir Crit Care Med. 2000;162:1957–63.
Sobin L. TNM classification of malignant tumors. 6th rev ed. New York: Wiley-Liss, 2002.
Yuan A, Yu CJ, Luh KT, et al. Aberrant p53 expression correlates with expression of vascular endothelial growth factor mRNA and interleukin-8 mRNA and neoangiogenesis in non-small-cell lung cancer. J Clin Oncol. 2002;20:900–10.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Sanchez-Mora N, Presmanes MC, Monroy V, et al. Micropapillary lung adenocarcinoma: a distinctive histologic subtype with prognostic significance. Case series. Hum Pathol. 2008;39:324–30.
Moon KC, Cho SY, Lee HS, et al. Distinct expression patterns of E-cadherin and beta-catenin in signet ring cell carcinoma components of primary pulmonary adenocarcinoma. Arch Pathol Lab Med. 2006;130:1320–5.
Prudkin L, Liu DD, Ozburn NC, et al. Epithelial-to-mesenchymal transition in the development and progression of adenocarcinoma and squamous cell carcinoma of the lung. Mod Pathol. 2009;22:668–78.
Disclosure
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Haro, A., Yano, T., Kohno, M. et al. Expression of Brachyury Gene Is a Significant Prognostic Factor for Primary Lung Carcinoma. Ann Surg Oncol 20 (Suppl 3), 509–516 (2013). https://doi.org/10.1245/s10434-013-2914-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-013-2914-9