Abstract
Background
Merkel cell carcinoma (MCC) is a rare, aggressive cutaneous neuroendocrine tumor usually occurring on sun-exposed skin in elderly patients. Clinical and pathologic factors associated with disease progression and mortality in patients with MCC are poorly defined. Recently, it has been reported that p63 expression in primary MCC is strongly associated with clinical outcome.
Methods
MCC patients diagnosed between July 1, 1993 and July 31, 2009 were identified from the surgical pathology records of the Sydney South West Area Health Service. Clinical, pathologic, treatment, and survival data were obtained and immunohistochemical analyses for p53, p63, and Ki-67 were performed. The associations of clinical and pathologic features with disease-free and disease-specific survival were analyzed.
Results
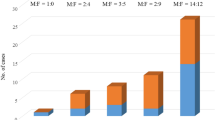
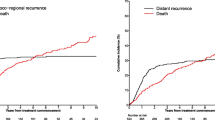
Ninety-five patients were identified (67 males, 28 females; median age at diagnosis of primary MCC 76 [range, 42–93] years). Increasing primary tumor thickness was significantly associated with poorer disease-free survival (5-year survival 18 % in tumors >10 mm thick compared with 69 % for patients with tumors ≤10 mm thick, p = 0.002) and disease-specific survival (5-year survival 74 % in tumors >10 mm thick compared with 97 % for patients with tumors ≤10 mm thick, p = 0.006). There was a strong positive correlation between the Ki-67 index (proportion of Ki-67-positive tumor nuclei) and tumor thickness (r = 0.39, n = 45, p = 0.008). Positive staining for p63 in MCC was infrequent (9 % of primary MCC) and showed no significant association with disease outcome.
Conclusions
Tumor thickness is significantly associated with disease-free survival in MCC. We recommend that primary tumor thickness be routinely recorded in the pathology reports of patients with primary MCC.


Similar content being viewed by others
References
Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107–10.
Bichakjian CK, Lowe L, Lao CD, et al. Merkel cell carcinoma: critical review with guidelines for multidisciplinary management. Cancer. 2007;110:1–12.
Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1–4.
Miller RW, Rabkin CS. Merkel cell carcinoma and melanoma: etiological similarities and differences. Cancer Epidemiol Biomarkers Prev. 1999;8(2):153–8.
Engels EA, Frisch M, Goedert JJ, et al. Merkel cell carcinoma and HIV infection. Lancet. 2002;359:497–8.
Busam KJ, Jungbluth AA, Rekthman N, et al. Merkel cell polyomavirus expression in Merkel cell carcinomas and its absence in combined tumors and pulmonary neuroendocrine carcinomas. Am J Surg Pathol. 2009;33:1378–85.
Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–100.
Garneski KM, Warcola AH, et al. Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J Invest Dermatol. 2009;129:246–8.
Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23(10):2300–9.
Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375–81.
Eng TY, Boersma MG, Fuller CD, et al. A comprehensive review of the treatment of Merkel cell carcinoma. Am J Clin Oncol. 2007;30(6):624–36.
Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. Merkel cell carcinoma. New York: Springer; 2010. p. 315–9.
Eng TY, Boersma MG, Fuller CD, et al. Treatment of merkel cell carcinoma. Am J Clin Oncol. 2004;27(5):510–5.
Pitale M, Sessions RB, Hussain S. An analysis of prognostic factors in cutaneous neuroendocrine carcinoma. Laryngoscope. 1992;102:244–9.
Shaw JH, Rumball E. Merkel cell tumour: clinical behaviour and treatment. Br J Surg. 1991;78:138–42.
Coit DG. Merkel cell carcinoma. Ann Surg Oncol. 2001;8(9 Suppl):99S–102S.
Skelton HG, Smith KJ, Hitchcock CL, et al. Merkel cell carcinoma: analysis of clinical, histologic, and immunohistologic features of 132 cases with relation to survival. J Am Acad Dermatol. 1997;37(5):734–9.
Llombart B, Monteagudo C, Lopez-Guerrero JA, et al. Clinicopathological and immunohistochemical analysis of 20 cases of Merkel cell carcinoma in search of prognostic markers. Histopathology. 2005;46:622–34.
Mott RT, Smoller BR, Morgan MB. Merkel cell carcinoma: a clinicopathologic study with prognostic implications. J Cutan Pathol. 2004;31:217–23.
Asioli S, Righi A, Volante M, et al. p63 expression as a new prognostic marker in Merkel cell carcinoma. Cancer. 2007;110:640–7.
Carson HJ, Reddy V, Taxy JB. Proliferation markers and prognosis in Merkel cell carcinoma. J Cutan Pathol. 1998;25(1):16–9.
Feinmesser M, Halpern M, Fenig E, et al. Expression of the apoptosis-related oncogenes bcl-2, bax, and p53 in Merkel cell carcinoma: can they predict treatment response and clinical outcome? Hum Pathol. 1999;30:1367–72.
Fernández-Figueras MT, Puig L, Musulén E, et al. Expression profiles associated with aggressive behavior in Merkel cell carcinoma. Mod Pathol. 2007;20:90–101.
Kennedy MM, Blessing K, King G, Kerr KM. Expression of bcl-2 and p53 in Merkel cell carcinoma. An immunohistochemical study. Am J Dermatopathol. 1996;18:273–7.
Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg Oncol. 1970;172:902–8.
Kerfoot C, Huang W, Rottenberg SA. Immunohistochemical analysis of advanced human breast carcinomas reveals downregulation of protein kinase C alpha. J Histochem Cytochem. 2004;52(3):419–22.
Fields RC, Busam KJ, Chou JF, et al. Five hundred patients with merkel cell carcinoma evaluated at a single institution. Ann Surg. 2011;254(3):465–75.
Andea AA, Coit DG, Amin B, Busam KJ. Merkel cell carcinoma: histologic features and prognosis. Cancer. 2008;113(9):2549–58.
Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–206.
Thompson JF, Soong SJ, Balch CM, et al. The prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional AJCC melanoma staging database. J Clin Oncol. 2011;29:2199–205.
Fernández-Figueras MT, Puig L, Musulen E, et al. Prognostic significance of p27Kip1, p45Skp2 and Ki67 expression profiles in Merkel cell carcinoma, extracutaneous small cell carcinoma, and cutaneous squamous cell carcinoma. Histopathology. 2005;46:614–21.
Koljonen V, Tukiainen E, Haglund C. Proliferative activity detected by Ki67 correlates with poor outcome in Merkel cell carcinoma. Histopathology. 2006;49(5):551–3.
Schmidt M, Janssen K, Dockhorn-Dworniczak B, et al. P53 abnormalities are rare events in neuroendocrine (Merkel cell) carcinoma of the skin. An immunohistochemical and SSCP analysis. Virchows Arch. 1997;430:233–7.
Asioli S, Righi A, de Biase D, et al. Expression of p63 is the sole independent marker of aggressiveness in localised (stage I–II) Merkel cell carcinomas. Mod Pathol. 2011;24:1451–61.
Allen PJ, Busam K, Hill AD, et al. Immunohistochemical analysis of sentinel lymph nodes from patients with Merkel cell carcinoma. Cancer. 2001;92:1650–5.
Hill AD, Brady MS, Coit DG. Intraoperative lymphatic mapping and sentinel lymph node biopsy for Merkel cell carcinoma. Br J Surg. 1999;86:518–21.
Warner RE, Quinn MJ, Hruby G, et al. Management of merkel cell carcinoma: the roles of lymphoscintigraphy, sentinel lymph node biopsy and adjuvant radiotherapy. Ann Surg Oncol. 2008;15:2509–18.
Gupta SG, Wang LC, Penas PF, et al. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: the Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142(6):685–90.
Mehrany K, Otley CC, Weenig RH, et al. A meta-analysis of the prognostic significance of sentinel lymph node status in Merkel cell carcinoma. Dermatol Surg. 2002;28:113–7.
Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population-based study. J Cutan Pathol. 2010;37:20–7.
Lemos BD, Storer BE, Iyer JG, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63(5):751–61.
Poulsen M, Round C, Keller J, et al. Factors influencing relapse-free survival in Merkel cell carcinoma of the lower limb—a review of 60 cases. Int J Radiat Oncol Biol Phys. 2010;76(2):393–7.
Guler-Nizam E, Leiter U, Metzler G, et al. Clinical course and prognostic factors of Merkel cell carcinoma of the skin. Br J Dermatol. 2009;161(1):90–4.
Ng L, Beer TW, Murray K. Vascular density has prognostic value in Merkel cell carcinoma. Am J Dermatopathol. 2008;30:442–5.
Beer TW, Ng LB, Murray K. Mast cells have prognostic value in Merkel cell carcinoma. Am J Dermatopathol. 2008;30:27–30.
Mojica P, Smith D, Ellenhorn JD. Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J Clin Oncol. 2007;25(9):1043–7.
Jabbour J, Cumming R, Scolyer RA, et al. Merkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage disease—results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann Surg Oncol. 2007;14(6):1943–52.
Eich HT, Eich D, Staar S, et al. Role of postoperative radiotherapy in the management of Merkel cell carcinoma. Am J Clin Oncol. 2002;25:50–6.
Medina-Franco H, Urist MM, Fiveash J, et al. Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol. 2001;8:204–8.
Boyle F, Pendlebury S, Bell D. Further insights into the natural history and management of primary cutaneous neuroendocrine (Merkel cell) carcinoma. Int J Radiat Oncol Biol Phys. 1995;31:315–23.
Acknowledgment
The authors are grateful to Ms. Robyn Soper and the laboratory staff (in particular Chitra de Silva and Trina Lum) at Royal Prince Alfred Hospital for preparation of the sections and immunohistochemical stains.
Disclosure
RAS is supported by the Cancer Institute NSW Clinical Research Fellowship Program. There are no other financial disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, C.S., Whalley, D., Haydu, L.E. et al. Increasing Tumor Thickness is Associated with Recurrence and Poorer Survival in Patients with Merkel Cell Carcinoma. Ann Surg Oncol 19, 3325–3334 (2012). https://doi.org/10.1245/s10434-012-2509-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-012-2509-x