Abstract
Background
Isolated limb infusion with melphalan (ILI-M) corrected for ideal body weight (IBW) is a well-tolerated treatment for patients with in-transit extremity melanoma with an approximate 29 % complete response (CR) rate. Sorafenib, a multi-kinase inhibitor, has been shown to augment tumor response to chemotherapy in preclinical studies.
Methods
A multi-institutional, dose-escalation, phase I study was performed to evaluate the safety and antitumor activity of sorafenib in combination with ILI-M. Patients with AJCC stage IIIB/IIIC/IV melanoma were treated with sorafenib starting at 400 mg daily for 7 days before and 7 days after ILI-M corrected for IBW. Toxicity, drug pharmacokinetics, and tumor protein expression changes were measured and correlated with clinical response at 3 months.
Results
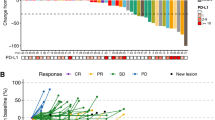
A total of 20 patients were enrolled at two institutions. The maximum tolerated dose (MTD) of sorafenib in combination with ILI-M was 400 mg. Four dose-limiting toxicities occurred, including soft tissue ulcerations and compartment syndrome. There were three CRs (15 %) and four partial responses (20 %). Of patients with the Braf mutation, 83 % (n = 6) progressed compared with only 33 % without (n = 12). Short-term sorafenib treatment did alter protein expression as measured with reverse phase protein array (RPPA) analysis, but did not inhibit protein expression in the MAP kinase pathway. Sorafenib did not alter melphalan pharmacokinetics.
Conclusion
This trial defined the MTD of systemically administered sorafenib in combination with ILI-M. Although some responses were seen, the addition of sorafenib to ILI-M did not appear to augment the effects of melphalan but did increase regional toxicity.





Similar content being viewed by others
References
Balch CM, Peters LJ. Cutaneous melanoma. In: De Vita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. 4 ed. Philadelphia: JB Lippincott; 1993. p. 1612–61.
Skene AI, Bulman AS, Williams TR, Thomas JM, Westbury G. Hyperthermic isolated perfusion with melphalan in the treatment of advanced malignant melanoma of the lower limb. Br J Surg. 1990;77:765–7.
Cornett WR, McCall LM, Petersen RP, Ross MI, Briele HA, Noyes RD, et al. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American college of surgeons oncology group trial Z0020. J Clin Oncol. 2006;24:4196–201.
Taber SW, Polk HC, Jr. Mortality, major amputation rates, and leukopenia after isolated limb perfusion with phenylalanine mustard for the treatment of melanoma. Ann Surg Oncol. 1997;4:440–5.
Vrouenraets BC, Nieweg OE, Kroon BB. Thirty-five years of isolated limb perfusion for melanoma: indications and results. Br J Surg. 1996;83:1319–28.
Lindner P, Doubrovsky A, Kam PC, Thompson JF. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002;9:127–36.
Beasley GM, Petersen RP, Yoo J, McMahon N, Aloia T, Petros W, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;15:2195–205.
Coleman A, Augustine CK, Beasley G, Sanders G, Tyler D. Optimizing regional infusion treatment strategies for melanoma of the extremities. Expert Rev Anticancer Ther. 2009;9:1599–609.
Beasley GM, Caudle A, Petersen RP, McMahon NS, Padussis J, Mosca PJ, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208:706–15; discussion 715–17.
Beasley GM, McMahon N, Sanders G, Augustine CK, Selim MA, Peterson B, et al. A phase 1 study of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with locally advanced in-transit malignant melanoma. Cancer. 2009;115:4766–74.
Hocker T, Tsao H. Ultraviolet radiation and melanoma: a systematic review and analysis of reported sequence variants. Hum Mutat. 2007;28:578–88.
Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–7.
Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–37.
Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–6.
Escudier B, Lassau N, Angevin E, Soria JC, Chami L, Lamuraglia M, et al. Phase I trial of sorafenib in combination with IFN alpha-2a in patients with unresectable and/or metastatic renal cell carcinoma or malignant melanoma. Clin Cancer Res. 2007;13:1801–9.
McDermott DF, Sosman JA, Gonzalez R, Hodi FS, Linette GP, Richards J, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 study group. J Clin Oncol. 2008;26:2178–85.
Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–21.
Augustine CK, Toshimitsu H, Jung SH, Zipfel PA, Yoo JS, Yoshimoto Y, et al. Sorafenib, a multikinase inhibitor, enhances the response of melanoma to regional chemotherapy. Mol Cancer Ther. 2010;9:2090–101.
Clark JW, Eder JP, Ryan D, Lathia C, Lenz HJ. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11:5472–80.
Awada A, Hendlisz A, Gil T, Bartholomeus S, Mano M, de Valeriola D, et al. Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005;92:1855–61.
Amaravadi RK, Schuchter LM, McDermott DF, Kramer A, Giles L, Gramlich K, et al. Phase II trial of temozolomide and sorafenib in advanced melanoma patients with or without brain metastases. Clin Cancer Res. 2009;15:7711–8.
Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–72.
Ehrsson H, Eksborg S, Lindfors A. Quantitative determination of melphalan in plasma by liquid chromatography after derivatization with N-acetylcysteine. J Chromatogr. 1986;380:222–8.
Zhao M, Rudek MA, He P, Hafner FT, Radtke M, Wright JJ, et al. A rapid and sensitive method for determination of sorafenib in human plasma using a liquid chromatography/tandem mass spectrometry assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;846:1–7.
Davies MA, Stemke-Hale K, Lin E, Tellez C, Deng W, Gopal YN, et al. Integrated molecular and clinical analysis of AKT activation in metastatic melanoma. Clin Cancer Res. 2009;15:7538–46.
Park ES, Rabinovsky R, Carey M, Hennessy BT, Agarwal R, Liu W, et al. Integrative analysis of proteomic signatures, mutations, and drug responsiveness in the NCI 60 cancer cell line set. Mol Cancer Ther. 2010;9:257–67.
Raymond A, Puri PK, Selim MA, Tyler DS, Nelson KC. Regional squamous cell carcinomas following systemic sorafenib and isolated limb infusion for regionally advanced metastatic melanoma of the limb. Arch Dermatol. 2010:146:1438–9.
McMahon N, Cheng TY, Beasley GM, Spasojevic I, Petros W, Augustine CK, et al. Optimizing melphalan pharmacokinetics in regional melanoma therapy: does correcting for ideal body weight alter regional response or toxicity? Ann Surg Oncol. 2009;16:953–61.
Beasley G, Riboh JC, Augustine CK, Zager JS, Hochwald SN, Grobmyer SR, et al. A prospective multi-center phase II trial of systemic ADH-1 in combination with melphalan via isolated limb infusion (M-ILI) in patients with advanced extremity melanoma. J Clin Oncol. 2011;29:1210–5.
Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8.
Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–44.
Arnault JP, Wechsler J, Escudier B, Spatz A, Tomasic G, Sibaud V, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27:e59–61.
Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;20:3960–7.
Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19.
Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–21.
Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–5.
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109.
Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9.
Jilaveanu L, Zito C, Lee SJ, Nathanson KL, Camp RL, Rimm DL, et al. Expression of sorafenib targets in melanoma patients treated with carboplatin, paclitaxel and sorafenib. Clin Cancer Res. 2009;15:1076–85.
Acknowledgment
Bayer provided drug only (sorafenib, Nexavar) for the phase I trial of systemic sorafenib and regional melphalan. This paper was supported in part by Duke University’s CTSA grant TL1RR024126 from NCRR/NIH (Coleman/Raymond), T32 grant CA093245-10 from NIH (Beasley), and the Duke Melanoma Research Fund (Tyler).
Author information
Authors and Affiliations
Corresponding author
Additional information
G.M. Beasley and A.P. Coleman contributed equally to this work.
Rights and permissions
About this article
Cite this article
Beasley, G.M., Coleman, A.P., Raymond, A. et al. A Phase I Multi-Institutional Study of Systemic Sorafenib in Conjunction with Regional Melphalan for In-Transit Melanoma of the Extremity. Ann Surg Oncol 19, 3896–3905 (2012). https://doi.org/10.1245/s10434-012-2373-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-012-2373-8