Abstract
Background
DOK2 is known as the substrate of chmeric p210bcr/abl oncoprotein characterizing chronic myelogenous leukemia with Philadelphia chromosome. Reduced DOK2 expression was recently reported in lung adenocarcinoma, suggesting that this protein acts as a tumor suppressor in solid tumors. The purpose of this study was to determine the significance of DOK2 in gastric cancer.
Methods
The study subjects were 118 patients who underwent curative surgery for gastric cancer, as well as 7 gastric cancer cell lines. The tissues and cell lines were analyzed for DOK2 gene and protein expressions by histopathology and immunohistochemistry, and also using a microsatellite marker for loss of heterozygosity. Correlation of survival with clinicopathological parameters was investigated by univariate and multivariate analyses.
Results
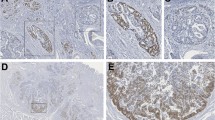
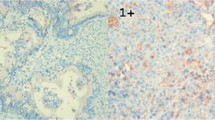
DOK2 expression was confirmed in the normal gastric mucosa. Considerable differences in the gene expression were noted among the gastric cell lines. Positive DOK2 expression was noted in the noncancerous regions of all pathological specimens, whereas 59 (50.0%) specimens of 118 patients were negatively stained in the tumor. Loss of heterozygosity was observed in 54.5% of DOK2(−) cases. DOK2(−) patients were more likely to develop recurrence than DOK2(+) and showed poorer 5-year overall survival (59.1%) than DOK2(+) (76.4%, P = .0403). Multivariate analysis identified pT (hazard ratio [HR] = 2.748, 95% confidence interval [95% CI] = 1.061–8.927, P = .0361), pN (HR = 2.486, 95% CI = 1.264–4.932, P = .0086), and DOK2(−) (HR = 2.343, 95% CI = 1.211–4.727, P = .0112) as significant and independent determinants of poor survival.
Conclusions
Our data suggest the potential usefulness of DOK2 as a marker of poor prognosis in patients with gastric cancer after curative resection.



Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Sobin LH, Gospodarowicz MK, Wittekind C, International Union against Cancer. TNM classification of malignant tumours. Chichester: Wiley-Blackwell; 2010.
Japanese Research Society for Gastric Cancer. Japanese classification of gastric carcinoma (in Japanese). 14th ed. Kyoto: Japanese Research Society for Gastric Cancer; 2010.
Zhang Y, Yan Z, Farooq A, Liu X, Lu C, Zhou MM, et al. Molecular basis of distinct interactions between Dok1 PTB domain and tyrosine-phosphorylated EGF receptor. J Mol Biol. 2004;343:1147–55.
Jones N, Dumont DJ. Recruitment of Dok-R to the EGF receptor through its PTB domain is required for attenuation of Erk/MAP kinase activation. Curr Biol. 1999;9:1057–60.
Van Slyke P, Coll ML, Master Z, Kim H, Filmus J, Dumont DJ. Dok-R mediates attenuation of epidermal growth factor-dependent mitogen-activated protein kinase and Akt activation through processive recruitment of c-Src and Csk. Mol Cell Biol. 2005;25:3831–41.
Zhao M, Janas JA, Niki M, Pandolfi PP, Van Aelst L. Dok-1 independently attenuates Ras/mitogen-activated protein kinase and Src/c-Myc pathways to inhibit platelet-derived growth factor-induced mitogenesis. Mol Cell Biol. 2006;26:2479–89.
Carpino N, Wisniewski D, Strife A, Marshak D, Kobayashi R, Stillman B, et al. P62(Dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88:197–204.
Jones N, Dumont DJ. The Tek/Tie2 receptor signals through a novel Dok-related docking protein, Dok-R. Oncogene. 1998;17:1097–108.
Dankort D, Jeyabalan N, Jones N, Dumont DJ, Muller WJ. Multiple ErbB-2/Neu phosphorylation sites mediate transformation through distinct effector proteins. J Biol Chem. 2001;276:38921–8.
Niki M, Di Cristofano A, Zhao M, Honda H, Hirai H, Van Aelst L, et al. Role of Dok-1 and Dok-2 in leukemia suppression. J Exp Med. 2004;200:1689–95.
Yasuda T, Shirakata M, Iwama A, Ishii A, Ebihara Y, Osawa M, et al. Role of Dok-1 and Dok-2 in myeloid homeostasis and suppression of leukemia. J Exp Med. 2004;200:1681–7.
Berger AH, Niki M, Morotti A, Taylor BS, Socci ND, Viale A, et al. Identification of Dok genes as lung tumor suppressors. Nat Genet. 2010;42:216–23.
Favre C, Gerard A, Clauzier E, Pontarotti P, Olive D, Nunes JA. DOK4 and DOK5: new Dok-related genes expressed in human T cells. Genes Immun. 2003;4:40–5.
Baffa R, Santoro R, Bullrich F, Mandes B, Ishii H, Croce CM. Definition and refinement of chromosome 8p regions of loss of heterozygosity in gastric cancer. Clin Cancer Res. 2000;6:1372–7.
Peng DF, Sugihara H, Mukaisho K, Tsubosa Y, Hattori T. Alterations of chromosomal copy number during progression of diffuse-type gastric carcinomas: metaphase- and array-based comparative genomic hybridization analyses of multiple samples from individual tumours. J Pathol. 2003;201:439–50.
Kong G, Oga A, Park CK, Kawauchi S, Furuya T, Sasaki K. DNA sequence copy number aberrations associated with histological subtypes and DNA ploidy in gastric carcinoma. Jpn J Cancer Res. 2001;92:740–7.
Sheffer M, Bacolod MD, Zuk O, Giardina SF, Pincas H, Barany F, et al. Association of survival and disease progression with chromosomal instability: a genomic exploration of colorectal cancer. Proc Natl Acad Sci USA. 2009;106:7131–6.
Brown MR, Chuaqui R, Vocke CD, Berchuck A, Middleton LP, Emmert-Buck MR, et al. Allelic loss on chromosome arm 8p: analysis of sporadic epithelial ovarian tumors. Gynecol Oncol. 1999;74:98–102.
Macoska JA, Trybus TM, Benson PD, Sakr WA, Grignon DJ, Wojno KD, et al. Evidence for three tumor suppressor gene loci on chromosome 8p in human prostate cancer. Cancer Res. 1995;55:5390–5.
El Gammal AT, Bruchmann M, Zustin J, Isbarn H, Hellwinkel OJ, Kollermann J, et al. Chromosome 8p deletions and 8q gains are associated with tumor progression and poor prognosis in prostate cancer. Clin Cancer Res. 2010;16:56–64.
Matsuyama H, Oba K, Matsuda K, Yoshihiro S, Tsukamoto M, Kinjo M, et al. Haploinsufficiency of 8p22 may influence cancer-specific survival in prostate cancer. Cancer Genet Cytogenet. 2007;174:24–34.
Stoehr R, Wissmann C, Suzuki H, Knuechel R, Krieg RC, Klopocki E, et al. Deletions of chromosome 8p and loss of sFRP1 expression are progression markers of papillary bladder cancer. Lab Invest. 2004;84:465–78.
Armes JE, Hammet F, de Silva M, Ciciulla J, Ramus SJ, Soo WK, et al. Candidate tumor-suppressor genes on chromosome arm 8p in early-onset and high-grade breast cancers. Oncogene. 2004;23:5697–702.
Chan KL, Lee JM, Guan XY, Fan ST, Ng IO. High-density allelotyping of chromosome 8p in hepatocellular carcinoma and clinicopathologic correlation. Cancer. 2002;94:3179–85.
Takada H, Imoto I, Tsuda H, Sonoda I, Ichikura T, Mochizuki H, et al. Screening of DNA copy-number aberrations in gastric cancer cell lines by array-based comparative genomic hybridization. Cancer Sci. 2005;96:100–10.
Chitale D, Gong Y, Taylor BS, Broderick S, Brennan C, Somwar R, et al. An integrated genomic analysis of lung cancer reveals loss of Dusp4 in EGFR-mutant tumors. Oncogene. 2009;28:2773–83.
Rubio-Moscardo F, Blesa D, Mestre C, Siebert R, Balasas T, Benito A, et al. Characterization of 8p21.3 chromosomal deletions in B-cell lymphoma: TRAIL-R1 and TRAIL-R2 as candidate dosage-dependent tumor suppressor genes. Blood. 2005;106:3214–222.
Seibold S, Rudroff C, Weber M, Galle J, Wanner C, Marx M. Identification of a new tumor suppressor gene located at chromosome 8p21.3-22. FASEB J. 2003;17:1180–2.
Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999;340:908–14.
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20.
Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–37.
Jin SS, Beak J, Kim H, Park J, Kim G, Kim D, et al. Prognostic factors in patients with recurrent gastric cancer after curative gastrectomy. J Clin Oncol. 2008;26:suppl;abstr 15612.
Hwang SE, Yang DH, Kim CY. Prognostic factors for survival in patients with hepatic recurrence after curative resection of gastric cancer. World J Surg. 2009;33:1468–72.
Lee CC, Lo SS, Wu CW, Shen KH, Li AF, Hsieh MC, et al. Peritoneal recurrence of gastric adenocarcinoma after curative resection. Hepatogastroenterology. 2003;50:1720–2.
Kerkar SP, Kemp CD, Avital I. Liver resections in metastatic gastric cancer. HPB (Oxford). 2010;12:589–96.
Sakamoto Y, Sano T, Shimada K, Esaki M, Saka M, Fukagawa T, et al. Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J Surg Oncol. 2007;95:534–9.
Kim HR, Cheon SH, Lee KH, Ahn JR, Jeung HC, Lee SS, et al. Efficacy and feasibility of radiofrequency ablation for liver metastases from gastric adenocarcinoma. Int J Hyperth. 2010;26:305–15.
An JY, Kim JY, Choi MG, Noh JH, Choi D, Sohn TS, et al. Radiofrequency ablation for hepatic metastasis from gastric adenocarcinoma. Yonsei Med J. 2008;49:1046–51.
Disclosures
Hiromichi Miyagaki and all authors of the manuscript: “Evaluation of DOK2 protein expression in gastric adenocarcinoma” declare no conflict of interest and no financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2011_2157_MOESM1_ESM.eps
Supplementary Figure 1. DOK genes expression in gastric mucosa and gastric cancer cell lines determined by RT-PCR. a Normal gastric mucosa cells were collected using laser microdissection. The expression of DOK1 and DOK2, but not DOK3, was evident in all normal gastric mucosa samples. The cDNA from peripheral mononuclear blood cells was used as a positive control. b In gastric cancer cell lines, DOK1 mRNA expression was observed in all gastric cancer cell line, while DOK3 mRNA expression was hardly observed. DOK2 expression was strong in MKN7 and AGS, weak in MKN74, and negative in MKN45, MKN45P, NUGC3, and RERFGC1B β2-microglobulin (B2M) was used as a control gene. (EPS 3,067 kb)
Rights and permissions
About this article
Cite this article
Miyagaki, H., Yamasaki, M., Takahashi, T. et al. DOK2 as a Marker of Poor Prognosis of Patients with Gastric Adenocarcinoma After Curative Resection. Ann Surg Oncol 19, 1560–1567 (2012). https://doi.org/10.1245/s10434-011-2157-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-011-2157-6