Abstract
Background
This study was designed to clarify retrospectively the clinical significance of occult metastases in both sentinel lymph nodes (SLNs) and non-SLNs in patients with early breast cancer.
Methods
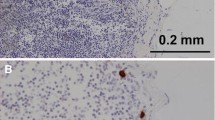
A total of 109 (80.1%) of 136 women with breast cancer who had consecutively undergone SLN biopsy (176 lymph nodes) were intraoperatively diagnosed as being free of SLN involvement. SLNs were routinely examined by hematoxylin–eosin (HE) staining of one to four frozen sections per node. Sixty-four (58.7%) of these patients also underwent backup axillary dissection. For the 109 patients, all formalin-fixed, paraffin-embedded tissues of SLNs and non-SLNs were entirely cut into 5-μm-thick sections. All serial step sections at 85-μm intervals were stained with HE and immunohistochemistry with pancytokeratin.
Results
Occult metastases in SLNs and non-SLNs were detected in 25 (23%) and 10 (16%) patients, respectively. The presence of occult SLN metastasis was marginally correlated with T-factor (P = 0.06), and predictive factors for occult non-SLN metastases were tumor nuclear grade (P = 0.039). With a median follow-up of 86 months, disease-free survival (P = 0.3) or overall survival (P = 0.8) did not differ between the patients with and without occult SLN metastases, regardless of backup axillary lymph node dissection.
Conclusions
SLN or non-SLN occult metastases detected by serial step sections at 85-μm intervals did not have significant prognostic implications.


Similar content being viewed by others
References
Fisher B, Bauer M, Wickerham DL, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer: an NSABP update. Cancer. 1983;52:1551–7.
Giuliano AE, Dale PS, Turner RR, Morton DL, Evans SW, Krasne DL. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;3:394–401.
Miltenburg DM, Miller C, Karamlou TB, Brunicardi FC. Meta analysis of sentinel lymph node biopsy in breast cancer. J Surg Res. 1999;84:138–42.
Cerni G, Gregori D, Merletti F, et al. Meta-analysis of non-sentinel node metastases associated with micrometastatic sentinel nodes in breast cancer. Br J Surg. 2004;91:1245–52.
Sobin LH, Gospodaroicz MK, Wittekind C (eds). International Union Against Cancer. TNM classification of malignant tumors. 7th ed. Oxford: Wiley-Blackwell; 2009. p. 12–5.
Tan LK, Giri D, Hummer AJ, et al. Occult axillary node metastases in breast cancer are prognostically significant: results in 368 node-negative patients with 20-year follow-up. J Clin Oncol. 2008;26:1803–9.
Chagpar A, Middleton LP, Sahin AA, et al. Clinical outcome of patients with lymph node-negative breast carcinoma who have sentinel lymph node micrometastases detected by immunohistochemistry. Cancer. 2005;103:1581–6.
Cox CE, Kiluk JV, Rikor AI, et al. Significance of sentinel lymph node micrometastases in human breast cancer: a SEER population-based analysis. J Am Coll Surg. 2008;206:261–8.
Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;366:2087–106.
Hansen NM, Grube B, Ye X, et al. Impact of micrometastases in the sentinel node of patients with invasive breast cancer. J Clin Oncol. 2009;27:4679–84.
de Boer M, van Deurzen CHM, van Dijck JAAM, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med. 2009;361:653–62.
Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–75.
Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–32.
Clark RM, Whelan T, Levine M, et al. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: an update. Ontario Clinical Oncology Group. J Natl Cancer Inst. 1996;88:1659–64.
Sato K, Tamaki K, Tsuda H, et al. Utility of axillary ultrasound examination to select breast cancer patients suited for optimal sentinel node biopsy. Am J Surg. 2004;187:679–83.
Cote RJ, Peterson HF, Chaiwun B, et al. Role of immunohistochemical detection of lymph-node metastases in management of breast cancer: International Breast Cancer Group. Lancet. 1999;354:869–900.
Dowlatshahi K, Fan M, Bloom KJ, et al. Occult metastases in the sentinel lymph nodes of patients with early stage breast carcinoma: A preliminary study. Cancer. 1999;86:990–6.
Van der Heiden-van der Loo M, Bezemer PD, Hennipman A, et al. Introduction of sentinel node biopsy and stage migration of breast cancer. Eur J Surg Oncol. 2006;32:710–4.
Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–53.
de Boer M, van Dijck JAAM, Bult P, Borm GF, Tjan-Heijnen VC G. Breast cancer prognosis and occult lymph node metastases, isolated tumor cells, and micrometastases. J Natl Cancer Inst. 2010;102:410–25.
Weaver DL, Ashikaga T, Krag DN, et al. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med. 2011;364:412–21.
Cote R, Giuliano AE, Hawes D, et al. ACOSOG Z0010: a multicenter prognostic study of sentinel node (SN) and bone marrow (BM) micrometastases in women with clinical T1/T2 N0 M0 breast cancer [abstract]. J Clin Oncol. 2010;28(Suppl):18.
Acknowledgment
We thank to Dr. Daisaku Morita and Ms. Yukiko Ohtsuka, National Defense Medical College, and Dr. Ken Shimizu and Ms. Motoko Korematsu, Saitama Social Insurance Hospital for technical help. The study was supported in part by grants from the Foundation for Promotion of Defense Medicine and from the grant for Cancer Research from the Ministry of Health, Labor, and Welfare, Japan.
Disclosures
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takeshita, T., Tsuda, H., Moriya, T. et al. Clinical Implications of Occult Metastases and Isolated Tumor Cells in Sentinel and Non-Sentinel Lymph Nodes in Early Breast Cancer Patients: Serial Step Section Analysis with Long-Term Follow-Up. Ann Surg Oncol 19, 1160–1166 (2012). https://doi.org/10.1245/s10434-011-2085-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-011-2085-5