Abstract
Background
This study utilized the imaging data of primary liver cancer (PLC) treated with floxuridine (FUDR) and bevacizumab to test the hypothesis that dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) parameters correlate with tissue hypoxia markers and treatment outcome.
Methods
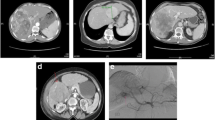
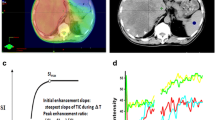
Seventeen patients with PLC were treated with hepatic artery infusional (HAI) FUDR for 14 days followed by systemic bevacizumab therapy. DCE-MRI images were obtained at baseline and after HAI FUDR and bevacizumab therapy. The parameters (Ktrans, AUC) pertaining to perfusion and vascular permeability of the tumor and adjacent liver parenchyma were measured with DCE-MRI. Tissue obtained at baseline was stained for hypoxia markers (anti-hypoxia inducible factor-1α, anti-carbonic anhydrase IX, and vascular endothelial growth factor). Changes in DCE-MRI parameters were correlated with tissue hypoxia and time to progression (TTP).
Results
The median TTP was 8.8 months. Significant decreases in AUC90 (P = 0.004), AUC180 (P = 0.004), and Ktrans (P = 0.05) were noted in tumors after bevacizumab but not in nontumor areas. TTP correlated inversely with changes in AUC90 and AUC180 after bevacizumab (P = 0.002 and P = 0.0001). Reductions in tumor perfusion (AUC90 and AUC180) were greater in tumors expressing anti-hypoxia inducible factor-1α (P = 0.02 and 0.03), vascular endothelial growth factor (P = 0.01 and P = 0.01), and anti-carbonic anhydrase IX (P = 0.009 and P = 0.009).
Conclusions
In patients with PLC, bevacizumab induces a reduction in tumor perfusion measured by DCE-MRI. These changes correlate with TTP and tissue markers of tumor hypoxia.



Similar content being viewed by others
References
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Kuo MD, Gollub J, Sirlin CB, Ooi C, Chen X. Radiogenomic analysis to identify imaging phenotypes associated with drug response gene expression programs in hepatocellular carcinoma. J Vasc Interv Radiol. 2007;18:821–31.
Segal E, Sirlin CB, Ooi C, et al. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat Biotechnol. 2007;25:675–80.
Diehn M, Nardini C, Wang DS, et al. Identification of noninvasive imaging surrogates for brain tumor gene-expression modules. Proc Natl Acad Sci USA. 2008;105:5213–8.
Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002;97:72–81.
Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91.
Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96.
Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006;24:3293–8.
Devries AF, Griebel J, Kremser C, et al. Tumor microcirculation evaluated by dynamic magnetic resonance imaging predicts therapy outcome for primary rectal carcinoma. Cancer Res. 2001;61:2513–6.
Hahn OM, Yang C, Medved M, et al. Dynamic contrast-enhanced magnetic resonance imaging pharmacodynamic biomarker study of sorafenib in metastatic renal carcinoma. J Clin Oncol. 2008;26:4572–8.
Bottaro DP, Liotta LA. Cancer: out of air is not out of action. Nature. 2003;423:593–5.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32.
Willett CG, Kozin SV, Duda DG, et al. Combined vascular endothelial growth factor–targeted therapy and radiotherapy for rectal cancer: theory and clinical practice. Semin Oncol. 2006;33:S35–40.
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–5.
Hobbs SK, Monsky WL, Yuan F, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95:4607–12.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62.
Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. 2006;24:5601–8.
Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol. 2009;27:3020–6.
Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38.
Kemeny N, Capanu M, D’Angelica M, et al. Phase I trial of adjuvant hepatic arterial infusion (HAI) with floxuridine (FUDR) and dexamethasone plus systemic oxaliplatin, 5-fluorouracil and leucovorin in patients with resected liver metastases from colorectal cancer. Ann Oncol. 2009;20:1236–41.
Allen PJ, Nissan A, Picon AI, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg. 2005;201:57–65.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Kemeny N, Cohen A, Seiter K, et al. Randomized trial of hepatic arterial floxuridine, mitomycin, and carmustine versus floxuridine alone in previously treated patients with liver metastases from colorectal cancer. J Clin Oncol. 1993;11:330–5.
Jarnagin WR, Schwartz LH, Gultekin DH, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20:1589–95.
Lockhart AC, Rothenberg ML, Dupont J, et al. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol. 2010;28:207–14
Kety SS. The theory and applications of the exchange of inert gas at the lungs and tissues. Pharmacol Rev. 1951;3:1–41.
Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–32.
Weinmann HJ, Laniado M, Mutzel W. Pharmacokinetics of GdDTPA/dimeglumine after intravenous injection into healthy volunteers. Physiol Chem Phys Med NMR. 1984;16:167–72.
Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. 1991;17:357–67.
Gönen M, Heller G. Concordance probability and discriminative power of proportional hazards regression. Biometrika. 2005;92:965–70.
Nakanuma Y, Harada K, Ishikawa A, Zen Y, Sasaki M. Anatomic and molecular pathology of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10:265–81.
Zhu AX, Sahani DV, Duda DG, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027–35.
Morgan B, Thomas AL, Drevs J, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol. 2003;21:3955–64.
Rosen MA, Schnall MD. Dynamic contrast-enhanced magnetic resonance imaging for assessing tumor vascularity and vascular effects of targeted therapies in renal cell carcinoma. Clin Cancer Res. 2007;13:770s–6s.
Koukourakis MI, Bentzen SM, Giatromanolaki A, et al. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol. 2006;24:727–35.
Hui EP, Chan AT, Pezzella F, et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res. 2002;8:2595–604.
Thelen A, Scholz A, Weichert W, et al. Tumor-associated angiogenesis and lymphangiogenesis correlate with progression of intrahepatic cholangiocarcinoma. Am J Gastroenterol. 2010;105:1123–32.
Acknowledgment
This work was supported by National Cancer Institute R21 CA121553-01A1 (WRJ).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yopp, A.C., Schwartz, L.H., Kemeny, N. et al. Antiangiogenic Therapy for Primary Liver Cancer: Correlation of Changes in Dynamic Contrast-Enhanced Magnetic Resonance Imaging with Tissue Hypoxia Markers and Clinical Response. Ann Surg Oncol 18, 2192–2199 (2011). https://doi.org/10.1245/s10434-011-1570-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-011-1570-1