Abstract
Background
Peritoneal carcinomatosis (PC) remains a dreaded clinical syndrome and a common evolution of gastrointestinal and ovarian cancers. In recent years, hyperthermic intraperitoneal chemotherapy (HIPEC) after cytoreductive surgery has emerged as a promising strategy in the management of PC. In this study, a novel paclitaxel (Pac) formulation was investigated for its toxicity and bioavailability during HIPEC compared with Taxol®.
Materials and Methods
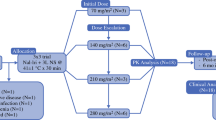
The maximum tolerated dose (MTD) after HIPEC of both formulations (Taxol® and Pac/RAME-β-CD) was determined. MTD was defined as the highest nonlethal dose with a reduction in body weight of ≤10% over 2 weeks. Blood parameters (red blood cell and white blood cell count, creatinine, ALT, and GGT) were evaluated over 20 days. Bioavailability of both Pac formulations after HIPEC was determined under normothermic (37°C) and hyperthermic (41°C) conditions for 90 min.
Results
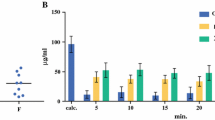
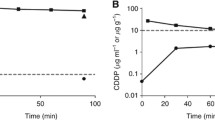
Following HIPEC, both formulations had a similar MTD: 0.24 mg paclitaxel per ml. Red blood cell count decreased to a minimum after 10 days and was not fully recovered after 20 days for both formulations. White blood cell monitoring showed a significant increase in neutrocytes at day 10 and 15 for the Pac/RAME-β-CD formulation. Liver and kidney parameters did not change significantly. Bioavailability data of Pac/RAME-β-CD showed a 40-fold increase of the area under the curve (AUC) of plasma concentrations compared with Taxol®. Hyperthermia yielded no significant differences in bioavailability data.
Conclusion
These results showed that both formulations had a similar toxicity profile but differed significantly in bioavailability.


Similar content being viewed by others
References
Koppe MJ, Boerman OC, Oyen WJG, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin—incidence and current treatment strategies. Ann Surg. 2006;243:212–22.
Tan DSP, Agarwal R, Kaye SB. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 2006;7:925–34.
McQuellon RP, Loggie BW, Fleming RA, Russel GB, Lehman AB, Rambo TD. Quality of life after intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal carcinomatosis. EJSO. 2001;27:65–73.
Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–32.
Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4:277–83.
Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellerman J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3:487–97.
Witkamp AJ, de Bree E, Van Goethem AR, Zoetmulder FAN. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev. 2001;27:365–74.
Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37:1590–8.
Bouquet W, Ceelen W, Fritzinger B, Pattyn P, Peeters M, Remon JP, et al. Paclitaxel/β-cyclodextrin complexes for hyperthermic peritoneal perfusion—formulation and stability. Eur J Pharm Biopharm. 2007;66:391–7.
Stokvis E, Ouwehand M, Nan LGAH, Kemper EM, van Tellingen O, Rosing H, et al. A simple and sensitive assay for the quantitative analysis of paclitaxel in human and Mouse plasma and brain tumor tissue using coupled liquid chromatography and tandem mass spectrometry. J Mass Spectrom. 2004;39:1506–12.
Allwood MC, Martin H. The extraction of diethylhexylphthalate (DEHP) from polyvinyl chloride components of intravenous infusion containers and administration sets by paclitaxel injection. Int J Pharm. 1996;127:65–71.
Baker HJ, Lindsey JR, Weisbroth SH. Laboratory Rat, Volume I: Biology and Disease. New York: Academic Press, 1979.
de Bree E, Rosing H, Filis D, et al. Cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy with paclitaxel: a clinical and pharmacokinetic study. Ann Surg Oncol. 2008;15:1183–92.
de Bree E, Theodoropoulos PA, Rosing H, Michalakis J, Romanos J, Beijnen JH, et al. Treatment of ovarian cancer using intraperitoneal chemotherapy with taxanes: From laboratory bench to bedside. Cancer Treat Rev. 2006,32;471–82.
Rajewski RA, Traiger G, Bresnahan J, Jaberaboansari P, Stella VJ. Preliminary safety evaluation of parenterally administered sulphoalkyl ether β-cyclodextrin derivatives. J Pharm Sci. 1995;84:927–32.
Rajewski RA, Stella VJ. Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery. J Pharm Sci. 1996;85:1142–69.
Frank DW, Gray JE, Weaver RN. Cyclodextrin nephrosis in the rat. Am J Pathol. 1976;83:367–74.
Irie T, Uekama K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J Pharm Sci. 1997;86:147–62.
Loftsson T, Duchêne D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329:1–11.
Bouquet W, Boterberg T, Ceelen W, Pattyn P, Peeters M, Bracke M, et al. In vitro cytotoxicity of paclitaxel/beta-cyclodextrin complexes for HIPEC. Int J Pharm. 2009;367:148–54.
Gelderblom H, Verweij J, van Zomeren DM, Buijs D, Ouwens L, Nooter K, et al. Influence of Cremophor EL on the bioavailability of intraperitoneal paclitaxel. Clin Canc Res. 2002;8:1237–41.
Sparreboom A, van Zuylen L, Brouwer E, Loos WJ, de Bruijn P, Gelderblom H, et al. Cremophor EL-mediated alteration of paclitaxel distribution in human blood: clinical pharmacokinetic implications. Cancer Res. 1999;59:1454–7.
Yokogawa K, Jin M, Furui N, Yamazaki M, Yoshihara H, Nomura M, et al. Disposition kinetics of taxanes after intraperitoneal administration in rats and influence of surfactant vehicles. J Pharm Pharmacol. 2004;56:629–34.
Dedrick RL, Flessner MF. Pharmacokinetic problems in peritoneal drug administration: tissue penetration and surface exposure. J Natl Cancer Inst. 1997;89:480–7.
Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–35.
Tsai M, Lu Z, Wang J, Yeh TK, Wientjes MG, Au JLS. Effects of carrier on disposition and antitumor activity of intraperitoneal paclitaxel. Pharm Res. 2007;24:1691–701.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bouquet, W., Ceelen, W., Adriaens, E. et al. In vivo Toxicity and Bioavailability of Taxol® and a Paclitaxel/β-Cyclodextrin Formulation in a Rat Model During HIPEC. Ann Surg Oncol 17, 2510–2517 (2010). https://doi.org/10.1245/s10434-010-1028-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-1028-x