Abstract
Background
Both activin A, a member of transforming growth factor β superfamily, and its inhibitor follistatin have been shown to be overexpressed in various cancers. We examined the potential role of activin A and follistatin in tissue and blood samples from patients with oral squamous cell carcinoma.
Methods
For activin A and follistatin, the expression of tissue samples from 92 patients was examined by immunohistochemical study, and the serum levels of blood samples from 111 patients and 91 healthy controls were measured by enzyme-linked immunosorbent assay.
Results
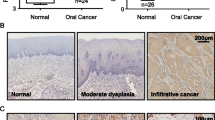
We found that overexpression of immunohistochemically detected activin A was correlated with positive N stage, poor histological differentiation, and perineural invasion (P = 0.029, 0.002, and 0.014, respectively). In survival analyses, patients with oral squamous cell carcinoma, whose tumors overexpressed activin A, had a worse prognosis for overall survival and disease-free survival (P = 0.009 and 0.007). However, expression of follistatin in tumor was not correlated with overall survival or disease-free survival. Serum activin A and follistatin levels in 111 untreated patients were neither significantly different from those of 91 control samples nor associated with any clinicopathological manifestations. In vitro suppression of activin A expression in OC3 cells using specific interfering RNA-attenuated cell proliferation, migration, and invasiveness.
Conclusions
These findings suggest that activin A overexpression in oral squamous cell carcinomas is associated with patients’ survival and may contribute to tumor progression and metastasis.





Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Reid BC, Winn DM, Morse DE, Pendrys DG. Head and neck in situ carcinoma: incidence, trends, and survival. Oral Oncol. 2000;36:414–20.
Boring CC, Squires TS, Tong T, Montgomery S. Cancer statistics, 1994. CA Cancer J Clin. 1994;44:7–26.
Silverman S, Jr. Demographics and occurrence of oral and pharyngeal cancers. The outcomes, the trends, the challenge. J Am Dent Assoc. 2001;132 Suppl:7S–11S.
Kodani I, Shomori K, Osaki M, Kuratate I, Ryoke K, Ito H. Expression of minichromosome maintenance 2 (MCM2), Ki-67, and cell-cycle-related molecules, and apoptosis in the normal-dysplasia-carcinoma sequence of the oral mucosa. Pathobiology. 2001;69:150–8.
Lippman SM, Sudbo J, Hong WK. Oral cancer prevention and the evolution of molecular-targeted drug development. J Clin Oncol. 2005;23:346–56.
Silverman S Jr., Gorsky M, Lozada F. (1984) Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer. 53:563–8.
Sudbo J. Novel management of oral cancer: a paradigm of predictive oncology. Clin Med Res. 2004;2:233–42.
Kingsley DM. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133–46.
Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta M, et al. Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin. Nature. 1986;321:779-82.
Vale W, Rivier J, Vaughan J, McClintock R, Corrigan A, Woo W, et al. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature. 1986;321:776–9.
Risbridger GP, Mellor SL, McPherson SJ, Schmitt JF. The contribution of inhibins and activins to malignant prostate disease. Mol Cell Endocrinol. 2001;180:149–53.
Risbridger GP, Schmitt JF, Robertson DM. Activins and inhibins in endocrine and other tumors. Endocr Rev. 2001;22:836–58.
Wildi S, Kleeff J, Maruyama H, Maurer CA, Buchler MW, Korc M. Overexpression of activin A in stage IV colorectal cancer. Gut. 2001;49:409–17.
Yoshinaga K, Mimori K, Yamashita K, Utsunomiya T, Inoue H, Mori M. Clinical significance of the expression of activin A in esophageal carcinoma. Int J Oncol. 2003;22:75–80.
Zheng W, Luo MP, Welt C, Lambert-Messerlian G, Sung CJ, Zhang Z, et al. Imbalanced expression of inhibin and activin subunits in primary epithelial ovarian cancer. Gynecol Oncol. 1998;69:23–31.
Thomas TZ, Wang H, Niclasen P, O’Bryan MK, Evans LW, Groome NP, et al. Expression and localization of activin subunits and follistatins in tissues from men with high grade prostate cancer. J Clin Endocrinol Metab.1997;82:3851–8.
Kleeff J, Ishiwata T, Friess H, Buchler MW, Korc M. Concomitant over-expression of activin/inhibin beta subunits and their receptors in human pancreatic cancer. Int J Cancer. 1998;77:860–8.
Seder CW, Hartojo W, Lin L, Silvers AL, Wang Z, Thomas DG, et al. Upregulated INHBA expression may promote cell proliferation and is associated with poor survival in lung adenocarcinoma. Neoplasia. 2009;11:388–96.
Reis FM, Cobellis L, Tameirao LC, Anania G, Luisi S, Silva IS, et al. Serum and tissue expression of activin a in postmenopausal women with breast cancer. J Clin Endocrinol Metab. 2002;87:2277–82.
Pirisi M, Fabris C, Luisi S, Santuz M, Toniutto P, Vitulli D, et al. Evaluation of circulating activin-A as a serum marker of hepatocellular carcinoma. Cancer Detect Prev. 2000;24:150–5.
Petraglia F, Florio P, Luisi S, Gallo R, Gadducci A, Vigano P, et al. Expression and secretion of inhibin and activin in normal and neoplastic uterine tissues. High levels of serum activin A in women with endometrial and cervical carcinoma. J Clin Endocrinol Metab. 1998;83:1194–200.
Coerver KA, Woodruff TK, Finegold MJ, Mather J, Bradley A, Matzuk MM. Activin signaling through activin receptor type II causes the cachexia-like symptoms in inhibin-deficient mice. Mol Endocrinol. 1996;10:534–43.
Di Simone N, Crowley WF, Jr., Wang QF, Sluss PM, Schneyer AL. Characterization of inhibin/activin subunit, follistatin, and activin type II receptors in human ovarian cancer cell lines: a potential role in autocrine growth regulation. Endocrinology 1996;137:486–94.
Yoshinaga K, Inoue H, Utsunomiya T et al. N-cadherin is regulated by activin A and associated with tumor aggressiveness in esophageal carcinoma. Clin Cancer Res. 2004;10:5702–7.
Yoshinaga K, Yamashita K, Mimori K, Tanaka F, Inoue H, Mori M. Activin a causes cancer cell aggressiveness in esophageal squamous cell carcinoma cells. Ann Surg Oncol. 2008;15:96–103.
Grusch M, Drucker C, Peter-Vorosmarty B, Erlach N, Lackner A, Losert A, et al. Deregulation of the activin/follistatin system in hepatocarcinogenesis. J Hepatol. 2006;45:673–80.
Matsuo SE, Leoni SG, Colquhoun A, Kimura ET. Transforming growth factor-beta1 and activin A generate antiproliferative signaling in thyroid cancer cells. J Endocrinol. 2006;190:141–50.
Reis FM, Luisi S, Carneiro MM, Cobellis L, Federico M, Camargos AF, et al. Activin, inhibin and the human breast. Mol Cell Endocrinol. 2004;225:77–82.
de Winter JP, ten Dijke P, de Vries CJ, van Achterberg TA, Sugino H, de Waele P, et al. Follistatins neutralize activin bioactivity by inhibition of activin binding to its type II receptors. Mol Cell Endocrinol. 1996;116:105–14.
Ogino H, Yano S, Kakiuchi S, Muguruma H, Ikuta K, Hanibuchi M, et al. Follistatin suppresses the production of experimental multiple-organ metastasis by small cell lung cancer cells in natural killer cell-depleted SCID mice. Clin Cancer Res. 2008;14:660–7.
Maeshima K, Maeshima A, Hayashi Y, Kishi S, Kojima I. Crucial role of activin a in tubulogenesis of endothelial cells induced by vascular endothelial growth factor. Endocrinology. 2004;145:3739–45.
Shimizu S, Seki N, Sugimoto T, Horiguchi S, Tanzawa H, Hanazawa T, et al. Identification of molecular targets in head and neck squamous cell carcinomas based on genome-wide gene expression profiling. Oncol Rep. 2007;18:1489–97.
Ye H, Yu T, Temam S, Ziober BL, Wang J, Schwartz JL, et al. Transcriptomic dissection of tongue squamous cell carcinoma. BMC Genomics. 2008;9:69.
Liao CT, Chang JT, Wang HM, Ng SH, Hsueh C, Lee LY, et al. Surgical outcome of T4a and resected T4b oral cavity cancer. Cancer. 2006;107:337–44.
De Marzo AM, Knudsen B, Chan-Tack K, Epstein JI. E-cadherin expression as a marker of tumor aggressiveness in routinely processed radical prostatectomy specimens. Urology. 1999;53:707–13.
Lin SC, Liu CJ, Chiu CP, Chang SM, Lu SY, Chen YJ. Establishment of OC3 oral carcinoma cell line and identification of NF-kappa B activation responses to areca nut extract. J Oral Pathol Med. 2004;33:79–86.
Liao CT, Huang SF, Chen IH, Chang JT, Wang HM, Ng SH, et al. Risk stratification of patients with oral cavity squamous cell carcinoma and contralateral neck recurrence following radical surgery. Ann Surg Oncol. 2009;16:159–70.
Simon DP, Vadakkadath Meethal S, Wilson AC, Gallego MJ, Weinecke SL, Bruce E, et al. Activin receptor signaling regulates prostatic epithelial cell adhesion and viability. Neoplasia. 2009;11:365–76.
Kang HY, Huang HY, Hsieh CY, Li CF, Shyr CR, Tsai MY, et al. Activin A enhances prostate cancer cell migration through activation of androgen receptor and is overexpressed in metastatic prostate cancer. J Bone Miner Res. 2009;24:1180–93.
Wagner K, Peters M, Scholz A, Benckert C, Ruderisch HS, Wiedenmann B, et al. Activin A stimulates vascular endothelial growth factor gene transcription in human hepatocellular carcinoma cells. Gastroenterol. 2004;126:1828–43.
Poulaki V, Mitsiades N, Kruse FE, Radetzky S, Iliaki E, Kirchhof B, et al. Activin a in the regulation of corneal neovascularization and vascular endothelial growth factor expression. Am J Pathol. 2004;164:1293–302.
Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28:1779–802.
Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25.
Kelber JA, Panopoulos AD, Shani G, Booker EC, Belmonte JC, Vale WW, et al. Blockade of Cripto binding to cell surface GRP78 inhibits oncogenic Cripto signaling via MAPK/PI3 K and Smad2/3 pathways. Oncogene. 2009;28:2324–36.
Schneyer AL, Sidis Y, Gulati A, Sun JL, Keutmann H, Krasney PA. Differential antagonism of activin, myostatin and growth and differentiation factor 11 by wild-type and mutant follistatin. Endocrinology 2008;149:4589–95.
Zhang L, Deng M, Parthasarathy R, Wang L, Mongan M, Molkentin JD, et al. MEKK1 transduces activin signals in keratinocytes to induce actin stress fiber formation and migration. Mol Cell Biol. 2005;25:60–5.
Yoshinaga K, Mimori K, Inoue H, Kamohara Y, Yamashita K, Tanaka F, et al. Activin A enhances MMP-7 activity via the transcription factor AP-1 in an esophageal squamous cell carcinoma cell line. Int J Oncol. 2008;33:453–9.
Do TV, Kubba LA, Antenos M, Rademaker AW, Sturgis CD, Woodruff TK. The role of activin A and Akt/GSK signaling in ovarian tumor biology. Endocrinology 2008;149:3809–16.
Acknowledgment
This study was supported by Chang Gung Memorial Hospital (Grant No. CMRPG360212 and CMRPG340481) and National Science Council (Grant No. NSC96-2314-B-182A-109-MY3), Taiwan.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
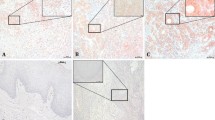
Supplementary Figure A
Overexpression of activin A and follistatin (FST) in breast and lung cancer tissues. Expression (brown staining) of activin A and FST indicated that the proteins were localized in the cytoplasm of breast and lung cancer cells (scale bar = 100 μm). (PPT 7666 kb)
Rights and permissions
About this article
Cite this article
Chang, KP., Kao, HK., Liang, Y. et al. Overexpression of Activin A in Oral Squamous Cell Carcinoma: Association with Poor Prognosis and Tumor Progression. Ann Surg Oncol 17, 1945–1956 (2010). https://doi.org/10.1245/s10434-010-0926-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-0926-2