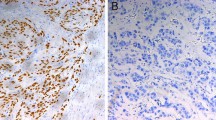
Abstract
Background
Response to chemotherapy and anatomical spread are significant prognostic factors in patients with esophageal squamous cell carcinoma (ESCC) treated by chemotherapy then surgery. Predicting the response to chemotherapy would allow significant optimization of cancer treatment.
Methods
Genomic mutation and protein expression of p53 were investigated retrospectively by polymerase chain reaction (PCR) single-strand conformation polymorphism (SSCP) and immunohistochemistry (IHC) using biopsy specimens from 77 ESCC patients before chemotherapy with 5-fluorouracil, adriamycin, and cisplatin. p53 status was correlated with various clinicopathological factors. Thereafter, we performed a prospective study of 20 consecutive patients to test our prediction model.
Results
The retrospective study showed mutant p53 genotype and positive p53 IHC staining in 46.8 and 55.8% of patients, respectively, which was not associated with patient’s clinicopathological findings including initial tumor stage. Objective response to chemotherapy was observed in 65.9% of patients with wild genotype, but in only 16.7% of patients with mutant genotype. Patients with mutations in p53 therefore showed significantly poorer prognosis than those without mutant p53. In contrast, p53 IHC staining did not correlate with response to chemotherapy, curative resection rate or prognosis. In the prospective study, p53 mutation was seen in 50% (10/20) of patients and was again consistently associated with poorer response to chemotherapy and poorer prognosis.
Conclusions
p53 genotype of pretreatment biopsy is a potentially useful predictor of response to chemotherapy and prognosis in ESCC patients. This information might be valuable to clinicians in deciding on the optimal clinical strategy in patients with ESCC.

Similar content being viewed by others
References
Burmeister BH, Denham JW, O’Brien M, et al. Combined modality therapy for esophageal carcinoma: preliminary results from a large Australasian multicenter study. Int J Radiat Oncol Biol Phys. 1995;32(4):997–1006.
Lee JL, Park SI, Kim SB, et al. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol. 2004;15(6):947–54.
Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339(27):1979–84.
Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8(3):226–34.
Roth JA, Pass HI, Flanagan MM, Graeber GM, Rosenberg JC, Steinberg S. Randomized clinical trial of preoperative and postoperative adjuvant chemotherapy with cisplatin, vindesine, and bleomycin for carcinoma of the esophagus. J Thorac Cardiovasc Surg. 1988;96(2):242–8.
Nygaard K, Hagen S, Hansen HS, et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg. 1992;16(6):1104–9; discussion 10.
Apinop C, Puttisak P, Preecha N. A prospective study of combined therapy in esophageal cancer. Hepatogastroenterology. 1994;41(4):391–3.
Le Prise E, Etienne PL, Meunier B, et al. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer. 1994;73(7):1779–84.
Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25(24):3719–25.
Maipang T, Vasinanukorn P, Petpichetchian C, et al. Induction chemotherapy in the treatment of patients with carcinoma of the esophagus. J Surg Oncol. 1994;56(3):191–7.
Law S, Fok M, Chow S, Chu KM, Wong J. Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus: a prospective randomized trial. J Thorac Cardiovasc Surg. 1997;114(2):210–7.
Malthaner RA, Collin S, Fenlon D. Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst Rev. 2006;3:CD001556.
Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337(3):161–7.
Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19(2):305–13.
Medical Research Council Oesophageal Cancer Working Party. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359(9319):1727–33.
Ancona E, Ruol A, Santi S, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer. 2001;91(11):2165–74.
Yano M, Takachi K, Doki Y, et al. Preoperative chemotherapy for clinically node-positive patients with squamous cell carcinoma of the esophagus. Dis Esophagus. 2006;19(3):158–63.
Harris CC. Structure and function of the p53 tumor suppressor gene: clues for rational cancer therapeutic strategies. J Natl Cancer Inst. 1996;88(20):1442–55.
Jacks T, Weinberg RA. Cell-cycle control and its watchman. Nature. 1996;381(6584):643–4.
Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74(6):957–67.
Sjostrom J, Blomqvist C, Heikkila P, et al. Predictive value of p53, mdm-2, p21, and mib-1 for chemotherapy response in advanced breast cancer. Clin Cancer Res. 2000;6(8):3103–10.
Bataille F, Rummele P, Dietmaier W, et al. Alterations in p53 predict response to preoperative high dose chemotherapy in patients with gastric cancer. Mol Pathol. 2003;56(5):286–92.
Kandioler-Eckersberger D, Ludwig C, Rudas M, et al. TP53 mutation and p53 overexpression for prediction of response to neoadjuvant treatment in breast cancer patients. Clin Cancer Res. 2000;6(1):50–6.
Kandioler-Eckersberger D, Kappel S, Mittlbock M, et al. The TP53 genotype but not immunohistochemical result is predictive of response to cisplatin-based neoadjuvant therapy in stage III non-small cell lung cancer. J Thorac Cardiovasc Surg. 1999;117(4):744–50.
Licitra L, Suardi S, Bossi P, et al. Prediction of TP53 status for primary cisplatin, fluorouracil, and leucovorin chemotherapy in ethmoid sinus intestinal-type adenocarcinoma. J Clin Oncol. 2004;22(24):4901–6.
Nakashima S, Natsugoe S, Matsumoto M, Kijima F, Takebayashi Y, Okumura H, et al. Expression of p53 and p21 is useful for the prediction of preoperative chemotherapeutic effects in esophageal carcinoma. Anticancer Res. 2000;20(3B):1933–7.
Ribeiro U, Jr., Finkelstein SD, Safatle-Ribeiro AV, et al. p53 sequence analysis predicts treatment response and outcome of patients with esophageal carcinoma. Cancer. 1998;83(1):7–18.
Kishi K, Doki Y, Miyata H, Yano M, Yasuda T, Monden M. Prediction of the response to chemoradiation and prognosis in oesophageal squamous cancer. Br J Surg. 2002;89(5):597–603.
Shiozaki H, Yano M, Tsujinaka T, et al. Lymph node metastasis along the recurrent nerve chain is an indication for cervical lymph node dissection in thoracic esophageal cancer. Dis Esophagus. 2001;14(3–4):191–6.
Yoshioka S, Fujiwara Y, Sugita Y, et al. Real-time rapid reverse transcriptase-polymerase chain reaction for intraoperative diagnosis of lymph node micrometastasis: clinical application for cervical lymph node dissection in esophageal cancers. Surgery. 2002;132(1):34–40.
Miyata H, Yano M, Doki Y, et al. A prospective trial for avoiding cervical lymph node dissection for thoracic esophageal cancers, based on intra-operative genetic diagnosis of micrometastasis in recurrent laryngeal nerve chain nodes. J Surg Oncol. 2006;93(6):477–84.
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16.
Urano N, Fujiwara Y, Doki Y, et al. Clinical significance of class III beta-tubulin expression and its predictive value for resistance to docetaxel-based chemotherapy in gastric cancer. Int J Oncol. 2006;28(2):375–81.
Hollstein MC, Metcalf RA, Welsh JA, Montesano R, Harris CC. Frequent mutation of the p53 gene in human esophageal cancer. Proc Natl Acad Sci USA. 1990;87(24):9958–61.
Kobayashi S, Koide Y, Endo M, Isono K, Ochiai T. The p53 gene mutation is of prognostic value in esophageal squamous cell carcinoma patients in unified stages of curability. Am J Surg. 1999;177(6):497–502.
Eastman A. Activation of programmed cell death by anticancer agents: cisplatin as a model system. Cancer Cells. 1990;2(8–9):275–80.
Lenz HJ, Hayashi K, Salonga D, et al. p53 point mutations and thymidylate synthase messenger RNA levels in disseminated colorectal cancer: an analysis of response and survival. Clin Cancer Res. 1998;4(5):1243–50.
Cabelguenne A, Blons H, de Waziers I, et al. p53 alterations predict tumor response to neoadjuvant chemotherapy in head and neck squamous cell carcinoma: a prospective series. J Clin Oncol. 2000;18(7):1465–73.
Fouret P, Temam S, Charlotte F, Lacau-St-Guily J. Tumour stage, node stage, p53 gene status, and bcl-2 protein expression as predictors of tumour response to platin-fluorouracil chemotherapy in patients with squamous-cell carcinoma of the head and neck. Br J Cancer. 2002;87(12):1390–5.
Temam S, Flahault A, Perie S, et al. p53 gene status as a predictor of tumor response to induction chemotherapy of patients with locoregionally advanced squamous cell carcinomas of the head and neck. J Clin Oncol. 2000;18(2):385–94.
Muro K, Ohtsu A, Boku N, et al. Association of p53 protein expression with responses and survival of patients with locally advanced esophageal carcinoma treated with chemoradiotherapy. Jpn J Clin Oncol. 1996;26(2):65–9.
Lam KY, Law S, Ma LT, Ong SK, Wong J. Pre-operative chemotherapy for squamous cell carcinoma of the oesophagus: do histological assessment and p53 overexpression predict chemo-responsiveness? Eur J Cancer. 1997;33(8):1221–5.
Shimada Y, Watanabe G, Yamasaki S, et al. Histological response of cisplatin predicts patients’ survival in oesophageal cancer and p53 protein accumulation in pretreatment biopsy is associated with cisplatin sensitivity. Eur J Cancer. 2000;36(8):987–93.
Fisher CJ, Gillett CE, Vojtesek B, Barnes DM, Millis RR. Problems with p53 immunohistochemical staining: the effect of fixation and variation in the methods of evaluation. Br J Cancer. 1994;69(1):26–31.
Wynford-Thomas D. P53 in tumour pathology: can we trust immunocytochemistry? J Pathol. 1992;166(4):329–30.
Finlay CA, Hinds PW, Tan TH, Eliyahu D, Oren M, Levine AJ. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988;8:531–9.
Hussain SP, Harris CC. Molecular epidemiology of human cancer: contribution of mutation spectra studies of tumor suppressor genes. Cancer Res. 1998;58:4023–37.
Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–9.
Bodner SM, Minna JD, Jensen SM, et al. Expression of mutant p53 proteins in lung cancer correlates with the class of p53 gene mutation. Oncogene. 1992;7:743–9.
O’Connor PM, Jackman J, Bae I, et al. Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 1997;57:4285–300.
Matsubara H, Kimura M, Sugaya M, et al. Expression of wild-type p53 gene confers increased sensitivity to radiation and chemotherapeutic agents in human esophageal carcinoma cells. Int J Oncol. 1999;14(6):1081–5.
Shimada H, Shimizu T, Ochiai T, et al. Preclinical study of p53 gene therapy for esopahgeal carcer. Surgery Today. 2001;31:597–604.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamasaki, M., Miyata, H., Fujiwara, Y. et al. p53 Genotype Predicts Response to Chemotherapy in Patients with Squamous Cell Carcinoma of the Esophagus. Ann Surg Oncol 17, 634–642 (2010). https://doi.org/10.1245/s10434-009-0851-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0851-4