Abstract
Background
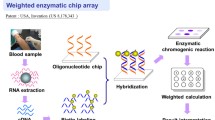
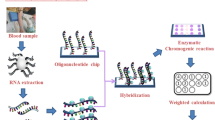
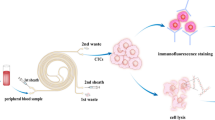
In 2008, the National Comprehensive Cancer Network suggested conducting a KRAS mutations test in metastatic colorectal cancer (mCRC) patients prior to administering therapy that uses anti-epidermal growth factor receptor (EGFR) monoclonal antibody. However, tests of KRAS mutations have been limited when traditional molecular techniques, such as polymerase chain reaction (PCR) combined direct sequencing, are used to obtain and analyze patients’ cancer tissues. If the primary tumor or metastatic tissues of patients with mCRC is unavailable, then such analysis will not be feasible. Our laboratory has successfully established a colorimetric membrane array analysis platform that could detect activating KRAS mutations from the peripheral blood of patients with various malignancies.
Methods
The current research aims to improve the above-mentioned technique not only by using chemiluminescence detection to replace color development, but also to add scores weighted according to the relevance of each gene to activating KRAS mutations.
Results
Our results show that the described weighted chemiluminescent membrane array (WCHMA) can detect circulating tumor cells (CTCs) harboring activating KRAS mutations in the peripheral blood in CRC. The sensitivity, specificity, and accuracy were 90.2, 94.9, and 93.5%, respectively, and the detection limitation was three colon tumor cells per millimeter of blood. The current study would significantly improve the detection sensitivity and accuracy over that of our previously designed membrane array method.
Conclusions
These findings also highlight the need to prompt further prospective studies on more cases of CRC to further establish the clinical relevance of activating KRAS mutation detection from peripheral blood in anti-EGFR-based chemotherapy that uses activating KRAS detection chips and the WCHMA analysis method.



Similar content being viewed by others
References
Weitz J, Koch M, Debus J, et al. Colorectal cancer. Lancet. 2005;365(9454):153–65.
Hurwitz HI, Yi J, Ince W, et al. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist. 2009;14(1):22–8.
Lenz HJ, Van Cutsem E, Khambata-Ford S, et al. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol. 2006;24(30):4914–21.
Giantonio BJ, Levy DE, O’Dwyer P J, et al. A phase II study of high-dose bevacizumab in combination with irinotecan, 5-fluorouracil, leucovorin, as initial therapy for advanced colorectal cancer: results from the Eastern Cooperative Oncology Group study E2200. Ann Oncol. 2006;17(9):1399–403.
Tabernero J, Van Cutsem E, Diaz-Rubio E, et al. Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2007;25(33):5225–32.
Berlin J, Posey J, Tchekmedyian S, et al. Panitumumab with irinotecan/leucovorin/5-fluorouracil for first-line treatment of metastatic colorectal cancer. Clin Colorectal Cancer. 2007;6(6):427–32.
Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(14):2311–9.
Arnold D, Hohler T, Dittrich C, et al. Cetuximab in combination with weekly 5-fluorouracil/folinic acid and oxaliplatin (FUFOX) in untreated patients with advanced colorectal cancer: a phase Ib/II study of the AIO GI Group. Ann Oncol. 2008;19(8):1442–9.
Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992–5.
Di Fiore F, Blanchard F, Charbonnier F, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer. 2007;96(8):1166–9.
Khambata-Ford S, Garrett CR, Meropol NJ, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25(22):3230–7.
De Roock W, Piessevaux H, De Schutter J, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19(3):508–15.
Lievre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26(3):374–9.
Santini D, Loupakis F, Vincenzi B, et al. High concordance of KRAS status between primary colorectal tumors and related metastatic sites: implications for clinical practice. Oncologist. 2008;13(12):1270–5.
Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(10):1626–34.
Van Cutsem E, Lang I, D’haens G, et al. KRAS status and efficacy in the first-line treatment of patients with metastatic colorectal cancer (mCRC) treated with FOLFIRI with or without cetuximab: the CRYSTAL experience. ASCO Annual Meeting 2008.
Bokemeyer C, Bondarenko I, Hartmann JT, et al. KRAS status and efficacy of first-line treatment of patients with metastatic colorectal cancer (mCRC) with FOLFOX with or without cetuximab: The OPUS experience. ASCO Annual Meeting 2008.
Artale S, Sartore-Bianchi A, Veronese SM, et al. Mutations of KRAS and BRAF in primary and matched metastatic sites of colorectal cancer. J Clin Oncol. 2008;26(25):4217–9.
Chen YF, Wang JY, Wu CH, et al. Detection of circulating cancer cells with K-ras oncogene using membrane array. Cancer Lett. 2005;229(1):115–22.
Yen LC, Yeh YS, Chen CW, et al. Detection of KRAS oncogene in peripheral blood as a predictor of the response to cetuximab plus chemotherapy in patients with metastatic colorectal cancer. Clin Cancer Res. 2009;15(13):4508–13.
International Union Against Cancer. TNM classification of malignant tumors. 6th ed. New York: Wiley-Liss; 2002.
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, New York; 1989. p. 6.22–6.34.
Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–9.
Wang JY, Yeh CS, Chen YF, et al. Development and evaluation of a colorimetric membrane-array method for the detection of circulating tumor cells in the peripheral blood of Taiwanese patients with colorectal cancer. Int J Mol Med. 2006;17(5):737–47.
Lin SR, Tsai JH, Yang YC, Lee SC. Mutations of K-ras oncogene in human adrenal tumours in Taiwan. Br J Cancer. 1998;77(7):1060–5.
Wang JY, Hsieh JS, Chen FM, et al. High frequency of activated K-ras codon 15 mutant in colorectal carcinomas from Taiwanese patients. Int J Cancer. 2003;107(3):387–93.
Wang JY, Hsieh JS, Lu CY, et al. The differentially mutational spectra of the APC, K-ras, and p53 genes in sporadic colorectal cancers from Taiwanese patients. Hepatogastroenterology. 2007;54(80):2259–65.
Guerrero S, Casanova I, Farre L, et al. K-ras codon 12 mutation induces higher level of resistance to apoptosis and predisposition to anchorage-independent growth than codon 13 mutation or proto-oncogene overexpression. Cancer Res. 2000;60(23):6750–6.
Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49(17):4682–9.
Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67(6):2643–8.
Lurje G, Nagashima F, Zhang W, et al. Polymorphisms in cyclooxygenase-2 and epidermal growth factor receptor are associated with progression-free survival independent of K-ras in metastatic colorectal cancer patients treated with single-agent cetuximab. Clin Cancer Res. 2008;14(23):7884–95.
Acknowledgment
This work was supported by grants from the National Science Council of the Republic of China (NSC 96-2320-B-037-011-MY3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ming-Je Yang and Hua-Hsien Chiu are contributed equally to this work
Jaw-Yuan Wang and Shiu-Ru Lin are contributed equally to this work
Rights and permissions
About this article
Cite this article
Yang, MJ., Chiu, HH., Wang, HM. et al. Enhancing Detection of Circulating Tumor Cells with Activating KRAS Oncogene in Patients with Colorectal Cancer by Weighted Chemiluminescent Membrane Array Method. Ann Surg Oncol 17, 624–633 (2010). https://doi.org/10.1245/s10434-009-0831-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0831-8