Abstract
Background
This study aimed to evaluate the usefulness of serial [18F] 2-fluoro-2-deoxy-d-glucose–positron emission tomography ([18F] FDG-PET) in potentially operable breast cancer with neoadjuvant chemotherapy.
Methods
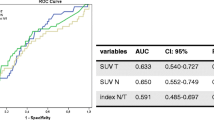
Serial positron emission tomography was undertaken in 66 breast cancer patients who comprised a subset of the population in a phase III randomized neoadjuvant trial at National Cancer Center, Korea. We assessed the peak standardized uptake value (SUVp) in the primary tumor and axillary nodes before and after neoadjuvant chemotherapy and calculated the reduction rate (RR) of the SUVp. By means of a receiver operating characteristic curve, we identified an optimal cutoff value for the RR for predicting the pathologic response and evaluated the prognostic power of this cutoff value.
Results
Ten patients (15.2%) experienced a pathologic complete response (pCR) in the primary tumor, and 19 patients (28.8%) experienced a pCR in the axillary nodes. The mean RR of the SUVp in primary tumors was 70.3% ± 28.7%, and this value was significantly different by the pathological response (89.2% ± 11.1% in pCR vs. 66.9% ± 29.6% in non-pCR, P < .001). When 84.8% of the RR was used as a cutoff value for the pCR, sensitivity and specificity was 70.0% and 69.6%, respectively. Ten patients (15.2%) developed recurrent disease at a median follow-up period of 61.5 (range, 13.5–71.8) months. In a univariate analysis, the 5-year disease-free survival (DFS) was correlated with the clinical T stage (91.1% in T1/2 vs. 71.4% in T3/4, P = .02), HER-2 status (77.8% in positive vs. 96.9% in negative, P = .03), and the 84.8% RR of the SUVp in the primary tumor (95.8% vs. 78.5%, P = .04). HER-2 positivity was a significant independent prognosticator in the multivariate analysis (hazard ratio 8.73, 95% confidence interval 1.03–73.84, P = .04). The presence of a pCR in the primary tumor or nodes was not a prognostic factor in this subset of patients. The RR of the SUVp in the axillary nodes was not correlated with the nodal pCR and DFS.
Conclusions
The RR of the SUVp in the primary tumor was correlated with the pathologic response and DFS. This study suggests the possible prognostic value of the RR in positron emission tomography by neoadjuvant chemotherapy.



Similar content being viewed by others
References
Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–93.
Wolff AC, Davidson NE. Primary systemic therapy in operable breast cancer. J Clin Oncol. 2000;18:1558–69.
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85.
Machiavelli MR, Romero AO, Perez JE, et al. Prognostic significance of pathological response of primary tumor and metastatic axillary lymph nodes after neoadjuvant chemotherapy for locally advanced breast carcinoma. Cancer J Sci Am. 1998;4:125–31.
Scholl SM, Fourquet A, Asselain B, et al. Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumours considered too large for breast conserving surgery: preliminary results of a randomised trial: S6. Eur J Cancer. 1994;30A:645–52.
Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–85.
Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–9.
Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85.
Gonzalez-Angulo AM, McGuire SE, Buchholz TA, et al. Factors predictive of distant metastases in patients with breast cancer who have a pathologic complete response after neoadjuvant chemotherapy. J Clin Oncol. 2005;23:7098–104.
Estevez LG, Gradishar WJ. Evidence-based use of neoadjuvant taxane in operable and inoperable breast cancer. Clin Cancer Res. 2004;10:3249–61.
Lee KS, Ro J, Nam BH, et al. A randomized phase-III trial of docetaxel/capecitabine versus doxorubicin/cyclophosphamide as primary chemotherapy for patients with stage II/III breast cancer. Breast Cancer Res Treat. 2008;109:481–9.
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Wahl RL, Zasadny K, Helvie M, et al. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol. 1993;11:2101–11.
Adler LP, Crowe JP, al-Kaisi NK, Sunshine JL. Evaluation of breast masses and axillary lymph nodes with [F-18] 2-deoxy-2-fluoro-d-glucose PET. Radiology. 1993;187:743–50.
Nieweg OE, Kim EE, Wong WH, et al. Positron emission tomography with fluorine-18-deoxyglucose in the detection and staging of breast cancer. Cancer. 1993;71:3920–5.
Smith IC, Welch AE, Hutcheon AW, et al. Positron emission tomography using [(18)F]-fluorodeoxy-d-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol. 2000;18:1676–88.
Schelling M, Avril N, Nahrig J, et al. Positron emission tomography using [(18)F]fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol. 2000;18:1689–95.
Abraham DC, Jones RC, Jones SE, et al. Evaluation of neoadjuvant chemotherapeutic response of locally advanced breast cancer by magnetic resonance imaging. Cancer. 1996;78:91–100.
Vinnicombe SJ, MacVicar AD, Guy RL, et al. Primary breast cancer: mammographic changes after neoadjuvant chemotherapy, with pathologic correlation. Radiology. 1996;198:333–40.
Chollet P, Charrier S, Brain E, et al. Clinical and pathological response to primary chemotherapy in operable breast cancer. Eur J Cancer. 1997;33:862–6.
Kaida H, Ishibashi M, Fujii T, et al. Improved detection of breast cancer on FDG-PET cancer screening using breast positioning device. Ann Nucl Med. 2008;22:95–101.
Duch J, Fuster D, Munoz M, et al. (18)F-FDG PET/CT for early prediction of response to neoadjuvant chemotherapy in breast cancer. Eur J Nucl Med Mol Imaging. [Epub ahead of print].
Kim SJ, Kim SK, Lee ES, et al. Predictive value of [18F]FDG PET for pathological response of breast cancer to neo-adjuvant chemotherapy. Ann Oncol. 2004;15:1352–7.
Emmering J, Krak NC, Van der Hoeven JJ, et al. Preoperative [18F] FDG-PET after chemotherapy in locally advanced breast cancer: prognostic value as compared with histopathology. Ann Oncol. 2008;19:1573–7.
Acknowledgment
This study was supported in part by National Cancer Center, Korea, grant 0610240.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jung, SY., Kim, SK., Nam, BH. et al. Prognostic Impact of [18F] FDG-PET in Operable Breast Cancer Treated with Neoadjuvant Chemotherapy. Ann Surg Oncol 17, 247–253 (2010). https://doi.org/10.1245/s10434-009-0710-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0710-3