Abstract
Background
RUNX3 is a major growth regulator of gastric epithelial cells that is involved in gastric tumorigenesis in both humans and mice. In this study, we investigated the involvement of RUNX3 in tumor progression, and in the prognosis of human gastric cancer.
Methods
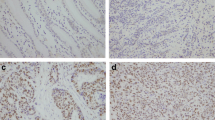
We analyzed the extent of RUNX3 protein expression by immunohistochemistry in 95 primary gastric adenocarcinomas, and correlated expression levels with clinicopathological parameters. We examined the effects of pFlag/RUNX3 on cell growth, apoptosis, and caspase-3 expression in AGS and SNU1 gastric cancer cell lines by colony-forming assay, terminal deoxynucleotidyl transferase (TdT)-mediate deoxyuridine triphosphatase (dUTP) nick-end labeling (TUNEL) assay, and Western blot analysis, respectively. The pFlag/RUNX3 effects on AGS invasion and migration potentials were also evaluated.
Results
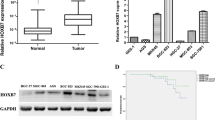
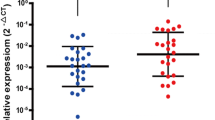
RUNX3 expression was lost in 37 (39%) cases of gastric cancer. The expression of RUNX3 in diffuse- and mixed-type cancers was less frequent than expression in intestinal-type cancer (P < 0.001 and P = 0.001, respectively). In addition, the loss of RUNX3 expression was associated with lymph node metastasis (P = 0.02), and correlated with poor gastric cancer survival (P = 0.018). The growth of gastric cancer cells was suppressed after pFlag/RUNX3 transfection. The re-expression of RUNX3 resulted in the upregulation of caspase-3 and promoted apoptosis. Furthermore, Re-expression of RUNX3 induced significant inhibitions of AGS cell invasion and migration in vitro.
Conclusions
This work shows that loss of RUNX3 expression is highly associated with lymph node metastasis and poor prognosis of gastric cancer. The re-expression of RUNX3 may induce apoptosis and inhibit the growth as well as invasion/migration of cancer cells. These results indicate that the targeting of the RUNX3 pathway could represent a potential modality for treating gastric cancer.





Similar content being viewed by others
References
Parkin DM, Pisani P, Fearly J. Estimates of worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993;54:594–606.
Chyou PH, Nomura AM, Hankin JH, et al. A case-cohort study of diet and stomach cancer. Cancer Res. 1990;50:7501–4.
Nomura A, Grove JS, Stemmermann GN, et al. A prospective study of stomach cancer and its relation to diet, cigarettes, and alcohol consumption. Cancer Res. 1990;50:627–31.
Inoue M, Tajima K, Hirose K, et al. Lifestyle and subsite of gastric cancer—joint effect of smoking and drinking habits. Int J Cancer. 1994;56:494–9.
El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402.
Watanabe T, Tada M, Nagai H, et al. Helicobacter pylori infection induces gastric cancer in Mongolian Gerbils. Gastroenterology. 1998;115:642–8.
Parorsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Eng J Med. 325: 1127–31, 1991.
Hsu PI, Lai KH, Chien AJ, et al. Impact of bacterial eradication on the cell proliferation and p53 protein accumulation in Helicobacter pylori-associated gastritis. Anticancer Res. 2000;20:1221–8.
Hsu PI, Hwang IR, Cittelly D, et al. Clinical presentation in relation to diversity within the Helicobacter pylori cag pathogenicity island. Am J Gastroenterol. 2002;97:2231–8.
Thompson GB, Van Heerden JA, Sarr MG. Adenocarcinoma of the stomach: are we making progress? Lancet. 1993;342:713–8.
Lauren, P. The two histological main types of gastric carcinoma. Diffuse and so-called intestinal type carcinoma. An attempt at histoclinical classification. Acta Pathol Microbiol Scand. 1965;64,31–49.
Tahra E. Molecular mechanism of stomach carcinogenesis. J Cancer Res Clin Oncol. 1993;119:265–72.
Wu MS, Shun CT, Wang HP, et al. Genetic alterations in gastric cancer: relation to hsitological subtypes, tumor stage, and Helicobacter pylori infection. Gastroenterology. 1997;112:1457–65.
Solcia E, Fiocca R, Luinetti O, et al. Intestinal and diffuse gastric cancers arise in a different background of Helicobacter pylori gastritis through different gene involvement. Am J Surg Path. 1996;20:S8–22.
Mayer B, Johnson JP, Leitl F, et al. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53:1690–5.
Chen HH, Chu RY, Hsu PN, et al. Loss of E-cadherin expression correlates with poor differentiation and invasion into adjacent organs in gastric adenocarcinomas. Cancer Lett. 2003;201:97–106.
Lund AH, van Lohuizen M. RUNX: a trilogy of cancer genes. Cancer Cell. 2002;1:213–5.
Li QL, Ito K, Sakakura C, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113–24.
Ito Y. Molecular basis of tissue-specific gene expression mediated by the runt domain transcription factor PEBP2/CBF. Genes Cells. 1999;4:685–96.
Yang S, Wei D, Wang D, et al. In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation. J Bone Miner Res. 2003;18:705–15.
Puig-Kroger A, Corbi A. RUNX3: a new player in myeloid gene expression and immune response. J Cell Biochem. 2006;98:744–56.
Kim WJ, Kim EJ, Jeong P, et al. RUNX3 inactivation by point mutations and aberrant DNA methylation in bladder tumors. Cancer Res. 2005;65:9347–54.
Ito K, Liu Q, Salto-Tellez M, et al. RUNX3, a novel tumor suppressor, is frequently inactivated in gastric cancer by protein mislocalization. Cancer Res. 2005;65:7743–50.
Chi XZ, Yang JO, Lee KY, et al. RUNX3 suppresses gastric epithelial cell growth by inducing p21(WAF1/Cip1) expression in cooperation with transforming growth factor β-activated SMAD. Mol Cell Biol. 2005;18:8097–107.
Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg. 1981;11:127–39.
Shen M, Stukenberg PT, Kirschner MW, et al. The essential mitotic peptidyl-prolyl isomerase Pin1 binds and regulates mitosis-specific phosphproteins. Genes Dev. 1998;12:706–20 .
Wang L, Wei D, Huang S, et al. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 2003;9:6371–80.
Kasagi N, Gomyo Y, Shirai H, et al. Apoptotic cell death in human gastric carcinoma: analysis by terminal deoxynuceotidyl transferase-mediated dUTP-biotin nick end labeling. Jpn J Cancer Res. 1994;85:939–45.
Wei D, Gong W, Oh SC, et al. Loss of RUNX3 expression significantly affects the clinical outcome of gastric cancer patients and its restoration causes drastic suppression of tumor growth and metastasis. Cancer Res. 2005;65:4809–16.
Moustakas A, Pardali K, Gaal A, et al. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol Lett. 2002;82:85–91.
Sakakura C, Hasegawa K, Miyagawa K, et al. A. Possible involvement of RUNX3 silencing in the peritoneal metastases of gastric cancers. Clin Cancer Res. 2005;11:6479–88.
Nagahama Y, Ishimaru M, Osaki M, et al. Apoptotic pathway induced by transduction of RUNX3 in the human gastric carcinoma cell line MKN-1. Cancer Sci. 2008;99:23–30.
Tahara E. Genetic pathways of tow types of gastric cancer. IARC Sci Publ. 2004;157:327–49.
Bae SC, Choi JK. Tumor suppressor activity of RUNX3. Oncogene. 2004;23:4336–40.
Acknowledgement
This work was supported in part by grants from the National Science Council (to P. I.Hsu, P. J. Lu, and M. H.); and Academia Sinica (to M. H.).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hsu, PI., Hsieh, HL., Lee, J. et al. Loss of RUNX3 Expression Correlates with Differentiation, Nodal Metastasis, and Poor Prognosis of Gastric Cancer. Ann Surg Oncol 16, 1686–1694 (2009). https://doi.org/10.1245/s10434-009-0428-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0428-2