Abstract
Background
An understanding of the methods of detection of recurrent melanoma after sentinel lymph node biopsy (SLNB) is essential for the coordination of a rational plan of follow-up.
Methods
Clinical stage I/II melanoma patients who underwent SLNB from 1991 to 2004 were identified from a prospectively maintained single-institution database. Detection of recurrence by self (awareness of symptoms or abnormal physical findings) or physician (discovered on routine physical or scheduled test) and timing of clinic visit were recorded. Postoperative follow-up included physical exam every 3–4 months for the first year, every 3–6 months for the second year, and every 6–12 months thereafter. Serum lactate dehydrogenase (LDH) and chest X-ray (CXR) were obtained annually. Computed tomography (CT) and positron emission tomography (PET) were performed selectively.
Results
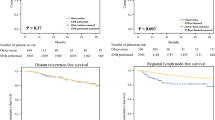
Of 1062 patients who underwent SLNB, 203 (19%) experienced 230 initial sites of recurrence; 198 patients were evaluable for follow-up. Median follow-up after first recurrence was 17 months. Symptoms and self-detected physical findings were present in 109 patients (55%); 85 patients (78%) were seen earlier than their scheduled visit. Self-detected physical findings identified in-transit (n = 26; 24%) and nodal (n = 25; 23%) disease. Physician detection occurred in 89 patients (45%), nearly half by a scheduled radiographic test (CXR, 16%; CT, 29%; PET, 1%). The method of detection significantly predicted post-recurrence survival (p < 0.05).
Conclusion
More than half of melanoma recurrences are self-detected; these patients have the most favorable post-recurrence survival rates because of the type of recurrence detected. The mode of detection is a significant predictor of post-recurrence survival. This supports an aggressive program of patient education in self-examination after SLNB for melanoma.

Similar content being viewed by others
References
Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001; 19:3635–48
Francken AB, Bastiaannet E, Hoekstra HJ. Follow-up in patients with localised primary cutaneous melanoma. Lancet Oncol 2005; 6:608–21
Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2007. CA Cancer J Clin 2007; 57:43–66
Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 1992; 127:392–9
Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol 1999; 17:976–83
Stadelmann W, Rapaport D, Soong S, et al. Prognostic clinical and pathologic features. 3rd edition. St. Louis: Quality Medical, 1998
Clary BM, Brady MS, Lewis JJ, et al. Sentinel lymph node biopsy in the management of patients with primary cutaneous melanoma: review of a large single-institutional experience with an emphasis on recurrence. Ann Surg 2001; 233:250–8
Gershenwald JE, Colome MI, Lee JE, et al. Patterns of recurrence following a negative sentinel lymph node biopsy in 243 patients with stage I or II melanoma. J Clin Oncol 1998; 16:2253–60
Gadd MA, Coit DG. Recurrence patterns and outcome in 1019 patients undergoing axillary or inguinal lymphadenectomy for melanoma. Arch Surg 1992; 127:1412–6
Dalal KM, Patel A, Brady MS, et al. Patterns of first recurrence and post-recurrence survival in patients with primary cutaneous melanoma after sentinel lymph node biopsy. Ann Surg Oncol 2007; 14:1934–42
Rigel DS, Friedman RJ, Kopf AQ. The incidence of malignant melanoma in the United States: issues as we approach the 21st century. J Am Acad Dermatol 1996; 34:839–47
Shumate CR, Urist MM, Maddox WA. Melanoma recurrence surveillance. Patient or physician based? Ann Surg 1995; 221:566–9
Baughan CA, Hall VL, Leppard BJ, et al. Follow-up in stage I cutaneous malignant melanoma: an audit. Clin Oncol 1993; 5:174–80
Weiss M, Loprinzi CL, Creagan ET, et al. Utility of follow-up tests for detecting recurrence disease in patients with malignant melanoma. JAMA 1995; 274:1703–5
Hofmann U, Szedlak M, Rittgen W., et al. Primary staging and follow-up in melanoma patients-monocenter evaulation of methods, costs, and patient survival. Br J Cancer 2002; 87:151–7
Wagner JD, Gordon MS, Chuang TY, et al. Predicting sentinel and residual lymph node basin disease after sentinel lymph node biopsy for melanoma. Cancer 2000; 89:453–62
Poo-Hwu WJ, Ariyan S, Lamb L, et al. Follow-up recommendations for patients with American Joint Committee on Cancer Stages I–III malignant melanoma. Cancer 1999; 86:2252–8
Essner R, Lee JH, Wanek LA, et al. Contemporary surgical treatment of advanced-stage melanoma. Arch Surg 2004; 139:961–6
Tsao H, Feldman M, Fullerton JF, et al. Early detection of asymptomatic pulmonary melanoma metastases by routine chest radiographs is not associated with improved survival. Arch Dermatol 2004; 140:67–70
Brobeil A, Rapaport D, Wells K, et al. Multiple primary melanomas: implications for screening and follow-up programs for melanoma. Ann Surg Oncol 1997; 4:19–23
Garbe C, Paul A, Kohler-Spath H, et al. Prospective evaluation of a follow-up schedule in cutaneous melanoma patients: recommendations for an effective follow-up strategy. J Clin Oncol 2003; 21:520–9
Ferrone CR, Ben Porat L, Panageas KS, et al. Clinicopathological features of and risk factors for multiple primary melanomas. JAMA 2005; 294:1647–54
Schofield PE, Beeney LJ, Thompson JF, et al. Hearing the bad news of a cancer diagnosis: the Australian melanoma patient’s perspective. Ann Oncol 2001; 12:365–71
Goggins WB, Tsao H. A population-based analysis of risk factors for a second primary cutaneous melanoma among melanoma survivors. Cancer 2003; 97:639–43
Johnson TM, Hamilton T, Lowe L. Multiple primary melanomas. J Am Acad Dermatol 1998; 39:422–7
Kang S, Barnhill RI, Mihm MR Jr, et al. Multiple primary cutaneous melanomas. Cancer 1992; 70:1911–6
Brandberg Y, Mansson-Brahme E, Ringborg U, et al. Psychological reactions in patients with malignant melanoma. Eur J Cancer 1995; 31:157–62
Mooney MM, Kulas M, McKinley B, et al. Impact on survival by method of recurrence detection in stage I and II cutaneous melanoma. Ann Surg Oncol 1998; 5:54–63
Mooney MM, Mettline C, Michalek Am, et al. Life-long screening of patients with intermediate-thickness cutaneous melanoma for asymptomatic pumonary recurrences: a cost-effectiveness analysis. Cancer 1997; 80:1052–64
NCCN. (2006) Melanoma. Clinical Practice Guidelines in Oncology, volume 2. pp 1–33
Rosselli del Truco M, Palli D, Cariddi A, et al. Intensive diagnostic follow-up after treatment of primary breast cancer: a randomized trial. National Research Council Project on Breast Cancer follow-up. JAMA 1994; 271:1593–7
Kersey PA, Iscoe NA, Gapski JA, et al. The value of staging and serial follow-up investigations in patients with completely resected, primary, cutaneous malignant melanoma. Br J Surg 1985; 72:614–7
Regan MW, Reid C, Griffiths RW, et al. Malignant melanoma evaluation of clinical follow-up by questionnaire survey. Br J Plast Surg 1985; 38:11–14
Basseres N, Grob JJ, Richard MA, et al. Cost-effectiveness of surveillance of stage I melanoma: a retrospective appraisal based on a 10-year experience in a dermatology department in France. Dermatology 1995; 191:199–203
Dicker TJ, Kavanagh G, Herd RM, et al. A rational approach to melanoma follow-up in patients with primary cutaneous melanoma. Br J Dermatol 1999; 140:249–54
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moore Dalal, K., Zhou, Q., Panageas, K.S. et al. Methods of Detection of First Recurrence in Patients with Stage I/II Primary Cutaneous Melanoma After Sentinel Lymph Node Biopsy. Ann Surg Oncol 15, 2206–2214 (2008). https://doi.org/10.1245/s10434-008-9985-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-008-9985-z