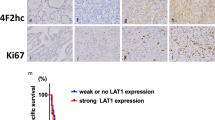
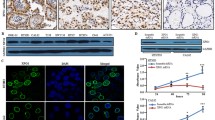
Abstract
Background
Medullary thyroid carcinoma (MTC) is a neuroendocrine malignancy that frequently metastasizes and has few treatments. This study was aimed at assessing the antitumor effects of suberoyl bishydroxamic acid (SBHA) in an in vivo model of MTC.
Methods
Nude mice were injected with human MTC cells, and the groups were treated with SBHA (200 mg/kg) or vehicle (dimethyl sulfoxide) in saline injection every other day for 12 days. Tumors were measured every 4 days and collected at 12 days for Western blot analysis.
Results
Treatment with SBHA resulted in an average 55% inhibition of tumor growth in the treatment group (P < .05). Analysis of SBHA-treated MTC tumors revealed a marked increase in the active form of Notch1 (NICD) with a concomitant decrease in achaete-scute complex-like 1 (ASCL1), a downstream target of Notch1 signaling, as well as the neuroendocrine tumor marker chromogranin A. Importantly, SBHA treatment resulted in an increase in protein levels of p21CIP1/WAF1, p27KIP1, cleaved caspase-9, cleaved caspase-3, and cleaved poly ADP-ribose polymerase and concomitant with a decrease in cyclin D1 and cyclin B1, indicating that the growth inhibition was due to both cell cycle arrest and apoptosis. Moreover, SBHA downregulated cell survival proteins Bcl-2 and Bcl-XL, but upregulated apoptotic proteins Bax, Bad, and Bmf.
Conclusion
These results demonstrate that SBHA inhibits MTC growth in vivo. SBHA is a promising candidate for further preclinical and clinical studies in MTC.





Similar content being viewed by others
References
Grozinsky-Glasberg S, Benbassat CA, Tsvetov G, et al. Medullary thyroid cancer: a retrospective analysis of a cohort treated at a single tertiary care center between 1970 and 2005. Thyroid 2007;17:549–56.
Greenblatt DY, Elson D, Mack E, et al. Initial lymph node dissection increases cure rates in patients with medullary thyroid cancer. Asian J Surg 2007;30:108–12.
Chen H, Roberts JR, Ball DW, et al. Effective long-term palliation of symptomatic, incurable metastatic medullary thyroid cancer by operative resection. Ann Surg 1998;227:887–95.
Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist 2008;13:539–47.
Mahlknecht U, Hoelzer D. Histone acetylation modifiers in the pathogenesis of malignant disease. Mol Med 2000;6:623–44.
Greenblatt DY, Cayo M, Ning L, et al. Suberoyl bishydroxamic acid inhibits cellular proliferation by inducing cell cycle arrest in carcinoid cancer cells. J Gastrointest Surg 2007;11:1515–20.
Gillespie S, Borrow J, Zhang XD, et al. Bim plays a crucial role in synergistic induction of apoptosis by the histone deacetylase inhibitor SBHA and TRAIL in melanoma cells. Apoptosis 2006;11:2251–65.
Neuzil J, Swettenham E, Gellert N. Sensitization of mesothelioma to TRAIL apoptosis by inhibition of histone deacetylase: role of Bcl-XL downregulation. Biochem Biophys Res Commun 2004;314:186–91.
Ning L, Greenblatt DY, Kunnimalaiyaan M, et al. Suberoyl bishydroxamic acid activates Notch1 signaling and induces apoptosis in medullary thyroid carcinoma cells. Oncologist 2008;13:98–104.
Leong SS, Hororszewicz JS, Shimaoka K, et al. A new cell line for study of human medullary thyroid carcinoma. In: Andreoli M, Monaco F, Robbins J (eds). Advances in Thyroid Neoplasia. Field Educational Italia, Rome, pp. 1981:95–108
Sippel RS, Carpenter JE, Kunnimalaiyaan M, et al. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol 2003;285:245–54.
Wang ZB, Liu YQ, Cui YF. Pathways to caspase activation. Cell Biol Int 2005;29: 489–96.
Miremadi A, Oestergaard MZ, Pharoah PD, et al. Cancer genetics of epigenetic genes. Hum Mol Genet 2007;16:28–49.
Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist 2007;12:535–42.
Greenblatt DY, Vaccaro A, Jaskula-Sztul R, et al. Valproic acid activates Notch1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist 2007;12:942–51.
Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, et al. Overexpression of the Notch1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem 2006;281:39819–30.
Kunnimalaiyaan M, Yan S, Wong F, et al. Hairy Enhancer of Split-1 (HES-1), a Notch1 effector, inhibits the growth of carcinoid tumor cells. Surgery 2005;138:1137–42.
Kunnimalaiyaan M, Traeger K, Chen H. Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol 2005;289:636–42.
Nakakura EK, Sriuranpong VR, Kunnimalaiyaan M, et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by Notch signaling. J Clin Endocrinol Metab 2005;90:4350–6.
Blumenschein G, Lu C, Kies M, et al. Phase II clinical trial of suberoylanilide hydroxamic acid (SAHA) in patients (pts) with recurrent and/or metastatic head and neck cancer (SCCHN). J Clin Oncol 2004;22:5578.
Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene 2007;26:1351–6.
Acknowledgments
Supported by American Cancer Society Research Scholars Grant 05-08301TBE; National Institutes of Health RO1 CA109053; NIH-R21CA117117; American College of Surgeons George H. A. Clowes Jr. Memorial Research Career Development Award; Vilas Foundation Research Grant; Carcinoid Cancer Foundation Research Award; Doctors Cancer Foundation Award; and the Society of Surgical Oncology Clinical Investigator Award. The authors thank Eric Wendt for editing the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ning, L., Jaskula-Sztul, R., Kunnimalaiyaan, M. et al. Suberoyl Bishydroxamic Acid Activates Notch1 Signaling and Suppresses Tumor Progression in an Animal Model of Medullary Thyroid Carcinoma. Ann Surg Oncol 15, 2600–2605 (2008). https://doi.org/10.1245/s10434-008-0006-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-008-0006-z