Abstract
Background
Patients with curatively resected colorectal cancer hepatic metastases often harbor occult metastatic disease and are at high risk of experiencing recurrence. This patient cohort is ideally suited to test novel therapies such as immunotherapy. We treated patients—post-hepatic resection—with anti-idiotype monoclonal antibody vaccines to the tumor-associated antigens carcinoembryonic antigen (CeaVac) and human milk fat globule (TriAb), both of which are co-expressed in more than 90% of colorectal cancer patients.
Methods
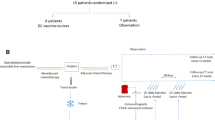
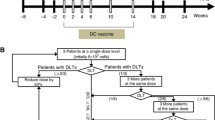
Vaccinations commenced 6–12 weeks post-hepatic resection and consisted of four biweekly treatments of 2 mg CeaVac and TriAb, then monthly treatments for 2 years, then on every other month for 3 years. The primary endpoint was to investigate the proportion of patients recurrence-free at 2 years, and the objective of the study was to demonstrate that at least 58% would be recurrence-free at this time to consider the regimen worthy of further study.
Results
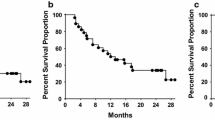
Between July 2001 and October 2004, 56 patients were accrued; 52 patients with margin-negative resection were eligible for analysis. Hepatic lobectomy was performed in 56% of patients with a median of one metastasis (range 1–3). Of the 52 eligible patients, 49 were evaluable for the primary end point. Median follow-up was 3.1 years. The proportion of patients recurrence-free at 2 years was 39%, with a lower confidence bound (LCB) of 0.29. Median recurrence-free survival was 16 months. The 2-year overall survival was 94% (95% CI, 0.81, 0.98). Only 10% of patients had documented grade-3 adverse events.
Conclusions
Anti-idiotype monoclonal antibody vaccine therapy with CeaVac and TriAb as an adjuvant to curative resection of colorectal cancer hepatic metastases is well tolerated but did not improve 2-year recurrence-free survival when compared with the expected value of 40% reported for hepatic resection alone.


Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin 2007;57(1):43–66
Scheele J, Stangl R, Attendorf-Hoffman A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 1990;77(11):1241–6
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350(23):2335–42
Lupinacci R, Penna C, Nordlinger B. Hepatectomy for resectable colorectal cancer metastases-indicators of prognosis, definition of resectability, techniques and outcomes. Surg Oncol Clin N Am 2007;16(3):493–506
Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg 2007;11(8):1057–77
Kornprat P, Jarnagin WR, Gonen M, et al. Outcome after hepatectomy for multiple (four or more) colorectal metastases in the era of effective chemotherapy. Ann Surg Oncol 2007; 19(3):1151–60
Jaeck D, Bachellier P, Guiguet M, et al. Long-term survival following resection of colorectal hepatic metastases. Association Francaise de Chirurgie. Br J Surg 1997;84(7):977–80
Fong Y, Cohen AM, Fortner JG. Liver resection for colorectal metastases. J Clin Oncol 1997;15(3):938–46
Portier G, Elias D, Bouche O, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol 2006;24(31):4976–82
Lindenmann J. Speculations on idiotypes and homobodies. Ann Immunol 1973;124(1):171–84
Jerne NK. Towards a network theory of the immune system. Ann Immunol 1974;125C(1–2):373–89
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81
Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 2004;240(4):644–58
Riethmuller G, Holz E, Schlimok G., et al. Monoclonal antibody therapy for resected Dukes’ C colorectal cancer: seven-year outcome of a multicenter randomized trial. J Clin Oncol 1998;16(5):1788–94
Colacchio TA, Niedzwiecki D, Camptor C, et al. Phase III trial of adjuvant immunotherapy with MOAb 17-1A following resection for stage II adenocarcinoma of the colon (CALGB 9581). Proc Am Soc Clin Oncol 2004; 23 (abstract 3522), p251
Harris JE, Ryan L, Hoover JC Jr, Stuart RK, et al. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: Eastern Cooperative Oncology Group Study E 5283. J Clin Oncol 2000;18(1):148–57
Vermorken JB, Claessen AM, van Titeren H, et al. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet 1999;353(9150):345–50
Foon KA, John WJ, Chakraborty M, et al. Clinical and immune responses in advanced colorectal cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics the carcinoembryonic antigen. Clin Cancer Res 1997;3(8):1267–76
Foon KA, John WJ, Chakraborty M, et al. Clinical and immune responses in resected colon cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics carcinoembryonic antigen. J Clin Oncol 1999;17(9):2889–95
Reece De Foon KA, Bhattarcharya-Chatterjee M. Use of the anti-idiotype breast cancer vaccine 11D10 in conjunction with autologous stem cell transplantation in patients with metastatic breast cancer. Clin Breast Cancer 2003;3(Suppl 4):S152–7
Acknowledgements
The research for CALGB 89903 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, MD, Chairman) and to the CALGB Statistical Center (Stephen George, PhD, CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. The following institutions participated in this study:
University of Chicago, Chicago, IL—Gini Fleming, M.D., supported by CA41287; CALGB Statistical Center, Duke University Medical Center, Durham, NC—Stephen George, Ph.D., supported by CA33601; University of California at San Francisco, San Francisco, CA—Alan P. Venook, M.D., supported by CA60138; University of North Carolina at Chapel Hill, Chapel Hill, NC—Thomas C. Shea, M.D., supported by CA47559; Dana-Farber Cancer Institute, Boston, MA—Eric P. Winer, M.D., supported by CA32291; The Ohio State University Medical Center, Columbus, OH—Clara D Bloomfield, M.D., supported by CA77658
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Presented in part at the 60th Annual Cancer Symposium of the Society of Surgical Oncology, March 15-18, 2007, Washington, DC
An erratum to this article can be found at http://dx.doi.org/10.1245/s10434-009-0466-9
Rights and permissions
About this article
Cite this article
Posner, M.C., Niedzwiecki, D., Venook, A.P. et al. A Phase II Prospective Multi-institutional Trial of Adjuvant Active Specific Immunotherapy Following Curative Resection of Colorectal Cancer Hepatic Metastases: Cancer and Leukemia Group B Study 89903. Ann Surg Oncol 15, 158–164 (2008). https://doi.org/10.1245/s10434-007-9654-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-007-9654-7