Abstract
Background
To assess the interaction between the expression of netrin-1 or of its receptors to the prognosis of ductal adenocarcinoma of the pancreas.
Methods
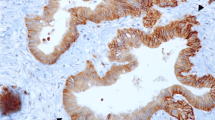
In 82 patients with resectable pancreatic adenocarcinoma who underwent curative operation, the expression patterns of netrin-1, deleted in colorectal carcinomas (DCC), UNC5H3, and neogenin were determined by immunohistochemical staining. Kaplan-Meier analysis was performed to assess the prognostic relevance of the examined expression patterns.
Results
Median follow-up was 15 ± 19.9 months (range, 4–108 months). Patients suffering from tumors with no or little expression of netrin-1 (n = 67) had a median recurrence-free survival of 10 months (95% CI, 7–13 months), while a middle to strong expression (n = 15) was associated with a significantly worse median recurrence-free survival of only four months (95% CI, three to five months, p = 0.0165). Overall and recurrence-free survival showed no significant differences between the different expression patterns of DCC, UNC5H3 or neogenin. Netrin-1 expression had significant impact (p = 0.001) on overall survival of patients suffering from poorly differentiated tumors. Stratification according to the nodal status revealed significant influence (p = 0.007) of UNC5H3 expression on the overall survival of patients with pN1 status.
Conclusion
Expression of netrin-1 has significant impact on time to tumor relapse in adenocarcinoma of the pancreas. Netrin-1 expression is associated with worse outcome in poorly differentiated pancreatic adenocarcinomas. Risk-stratification according to the UNC5H3 receptor expression pattern shows that node positive patients (pN1) with no to little UNC5H3 expression carry a significantly worse prognosis than those with middle to strong UNC5H3 expression.




Similar content being viewed by others
REFERENCES
Kennedy TE, Serafini T, de la Torre JR, et al. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 1994; 78:425–35
Yee KT, Simon HH, Tessier-Lavigne M, et al. Extension of long leading processes and neuronal migration in the mammalian brain directed by the chemoattractant netrin-1. Neuron 1999; 24:607–22
Deiner MS, Kennedy TE, Fazeli A, et al. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron 1997; 19:575–89
Ellezam B, Selles-Navarro I, Manitt C, et al. Expression of netrin-1 and its receptors DCC and UNC-5H2 after axotomy and during regeneration of adult rat retinal ganglion cells. Exp Neurol 2001; 168:105–15
Park KW, Crouse D, Lee M, et al. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci U S A 2004; 101:16210–5
Kaur B, Brat DJ, Devi NS, et al. Vasculostatin, a proteolytic fragment of brain angiogenesis inhibitor 1, is an antiangiogenic and antitumorigenic factor. Oncogene 2005; 24:3632–42
Bayliss PE, Bellavance KL, Whitehead GG, et al. Chemical modulation of receptor signaling inhibits regenerative angiogenesis in adult zebrafish. Nat Chem Biol 2006; 2:265–73
Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med 2002; 347:1593–603
Cutcliffe C, Kersey D, Huang CC, et al. Clear cell sarcoma of the kidney: up-regulation of neural markers with activation of the sonic hedgehog and Akt pathways. Clin Cancer Res 2005; 11:7986–94
Gavert N, Conacci-Sorrell M, Gast D, et al. L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol 2005; 168:633–42
Dunn JR, Reed JE, du Plessis DG, et al. Expression of ADAMTS-8, a secreted protease with antiangiogenic properties, is downregulated in brain tumours. Br J Cancer 2006; 94:1186–93
Wente MN, Keane MP, Burdick MD, et al. Blockade of the chemokine receptor CXCR2 inhibits pancreatic cancer cell-induced angiogenesis. Cancer Lett, 2006
Thiebault K, Mazelin L, Pays L, et al. The netrin-1 receptors UNC5H are putative tumor suppressors controlling cell death commitment. Proc Natl Acad Sci U S A 2003; 100:4173–8
Miyake K, Inokuchi K, Dan K, et al. Alterations in the deleted in colorectal carcinoma gene in human primary leukemia. Blood 1993; 82:927–30
Miyake S, Nagai K, Yoshino K, et al. Point mutations and allelic deletion of tumor suppressor gene DCC in human esophageal squamous cell carcinomas and their relation to metastasis. Cancer Res 1994; 54:3007–10
Thompson AM, Morris RG, Wallace M, et al. Allele loss from 5q21 (APC/MCC) and 18q21 (DCC) and DCC mRNA expression in breast cancer. Br J Cancer 1993; 68:64–8
Ekstrand BC, Mansfield TA, Bigner SH, et al. DCC expression is altered by multiple mechanisms in brain tumours. Oncogene 1995; 11:2393–402
Brewster SF, Gingell JC, Browne S, et al. Loss of heterozygosity on chromosome 18q is associated with muscle-invasive transitional cell carcinoma of the bladder. Br J Cancer 1994; 70:697–700
Lee JE, Kim HJ, Bae JY, et al. Neogenin expression may be inversely correlated to the tumorigenicity of human breast cancer. BMC Cancer 2005; 5:154
Fearon ER, Cho KR, Nigro JM, et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science 1990; 247:49–56
Fazeli A, Dickinson SL, Hermiston ML, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature 1997; 386:796–804
Mehlen P, Rabizadeh S, Snipas SJ, et al. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 1998; 395:801–4
Arakawa H. Netrin-1 and its receptors in tumorigenesis. Nat Rev Cancer 2004; 4:978–87
Mazelin L, Bernet A, Bonod-Bidaud C, et al. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature 2004; 431:80–4
Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990; 61:759–67
Klein JP, Moeschberger ML. Survival Analysis Techniques for Censored and Truncated data. New York, Springer, 1997
Cox DR. Regression models and life tables (with discussion). J R Statist Soc: 1972; 187–220
Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004; 350:1200–10
De Breuck S, Lardon J, Rooman I, et al. Netrin-1 expression in fetal and regenerating rat pancreas and its effect on the migration of human pancreatic duct and porcine islet precursor cells. Diabetologia 2003; 46:926–33
Wang RN, Kloppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia 1995; 38:1405–11
Rooman I, Lardon J, Bouwens L. Gastrin stimulates beta-cell neogenesis and increases islet mass from transdifferentiated but not from normal exocrine pancreas tissue. Diabetes 2002; 51:686–90
Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med 1993; 328:1433–7
ACKNOWLEDGMENTS
Financial support for this study was provided by a research grant from the Hamburger Krebsgesellschaft e. V.
We thank Petra Merkert, Antje Heineke, and Petra Schröder for excellent technical assistance. We are grateful to Susanne Pohl for her administrative support in the follow up of patients.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Link, BC., Reichelt, U., Schreiber, M. et al. Prognostic Implications of Netrin-1 Expression and Its Receptors in Patients with Adenocarcinoma of the Pancreas. Ann Surg Oncol 14, 2591–2599 (2007). https://doi.org/10.1245/s10434-007-9469-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-007-9469-6