Abstract
Background/Aim
Perioperative administration of immunoenriched diets attenuates the perioperative inflammatory response and reduces postoperative infection complications. However, many questions still remain unresolved in this area, such as the length of diet administration, diet composition, and the mechanisms involved. We performed an open, randomized, triple-arm study comparing the effect of two perioperative feeding regimens with a postoperative one.
Methods
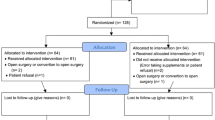
46 candidates for major elective surgery for malignancy in the upper gastrointestinal tract were randomized to drink preoperatively either 1 L of an immunoenriched formula (Impact) for 5 days (IEF group) or 1 L of Impact plus (Impact enriched with glycine) for 2 days (IEF plus group). The same product as the patient received preoperatively was given to both groups for 7 days postoperatively. In the control group (CON group), patients only received Impact for 7 days postoperatively; there was no preoperative treatment. The main outcome measures were postoperative C-reactive protein (CRP) serum levels.
Results
In the two preoperatively supplemented groups (treatment groups), perioperative endotoxin levels, CRP (postoperative day 7), and TNF-α (postoperative days 1 and 3) levels were significantly lower compared to the CON group (p < .01). Furthermore, the length of postoperative IMU/ICU stay (Impact 1.9 ± 1.3 days; Impact plus 2.2 ± 1.1 days; control group 5.9 ± 0.8 days) and length of hospital stay (Impact 19.7 ± 2.3 days; Impact plus 20.1 ± 1.3 days; control group 29.1 ± 3.6 days) were both reduced in the treatment groups compared to the control group. Infectious complications (Impact 2/14 (14%); Impact plus 5/17 (29%); control group 10/15 (67%)) also showed a trend toward reduction in the treatment groups.
Conclusions
Perioperative administration of an immunoenriched diet significantly reduces systemic perioperative inflammation and postoperative complications in patients undergoing major abdominal cancer surgery, when compared with postoperative diet administration alone. A shortened preoperative feeding regimen of 2 days with Impact enriched with glycine (Impact plus) was as effective as Impact administered for 5 days preoperatively.


Similar content being viewed by others
Abbreviations
- ASA:
-
American Society of Anesthesiologists
- CON group:
-
Control group
- CRP:
-
C-reactive protein
- ELISA:
-
Enzyme-linked immunosorbent assay
- ICU:
-
Intensive care unit
- IEF group:
-
Immunoenriched formula group
- IEF plus group:
-
Immunoenriched formula plus glycine group
- IL-6/8:
-
Interleukin 6/8
- IMC:
-
Intermediate care unit
- IV:
-
Intravenous
- LOS:
-
Length of hospital stay
- MODS:
-
Multiorgan dysfunction syndrome
- POD:
-
Postoperative day
- RNA:
-
Ribonucleic acid
- SD:
-
Standard deviation
- SIRS:
-
Systemic inflammatory response syndrome
- TNF-α:
-
Tumor necrosis factor alpha
- WBC:
-
White blood count
REFERENCES
Braga M, Gianotti L, Radaelli G, Vignali A, Mari G, Gentilini O, Di Carlo V. Perioperative immunonutrition in patients undergoing cancer surgery: results of a randomized double-blind phase 3 trial. Arch Surg 1999; 134:428–433
Senkal M, Zumtobel V, Bauer KH, Marpe B, Wolfram G, Frei A, Eickhoff V, Kemen M. Outcome and cost effectiveness of perioperative enteral immunonutrition in patients with elective upper gastrointestinal surgery: A prospective randomised study. Arch Surg 1999; 134:1309–16
Gianotti L, Braga M, Frei A, Greiner R, Di Carlo V. Health care resources consumed to treat postoperative infections: cost saving by perioperative immunonutrition. Shock 2000; 14:325–330
Tepaske R, Velthuis H, Oudemans-van Straaten HM, Heisterkamp SH, van Deventer SJ, Ince C, Eysman L, Kesecioglu J. Effect of preoperative oral immune-enhancing nutritional supplement on patients at high risk of infection after cardiac surgery: a randomized placebo-controlled trial. Lancet 2001; 358:696–701
Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology 2002; 122:1763–70
Pscheidl E, Schywalsky M, Tschaikowsky K, Böke-Pröls T. Fish oil-supplemented parenteral diets normalize splanchnic blood flow and improve killing of translocated bacteria in a low-dose endotoxin rat model. Crit Care Med 2000; 28:1489–96
Weimann A, Jauch K.W, Kemen M, Hiesmayr J.M, Horbach T, Kuse E.R, Vestweber K.H. DGEM-Leitlinie Enterale Ernährung: Chirurgie und Transplantation. Aktuel Ernaehr Med 2003; 28:51–60
Zhong Z, Wheeler MD, Froh M, Schemmer P, Yin M, Bunzendahl H, Bradford BU, Lemasters JJ. L-Glycine: a novel anti-inflammatory, immunomodulatory, and cytoprotective agent. Curr opin Clin Nutr Metab Care 2003; 6:229–40
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988; 16:128–40
Berger D, Schmidt UM, Otto S, Seidelmann M, Martin R, Beger HG. Incidence of pathophysiological relevance of postoperative endotoxemia. FEMS Immunolog Med Microbiol 1995; 11:285–90
Roumen RM, Frieling JT, van Tits HW, et al. Endotoxemia after major vascular operations. J Vasc Surg 1993; 18:853–7
Soong CV, Blair PH, Halliday MI, et al. Endotoxemia, the generation of cytokines and their relationship in intramucosal acidosis of the sigmoid colon in elective abdominal aneurysm repair. Eur J Vasc Surg 1993; 14:534–9
Buttenschoen K, Buttenschoen DC, Berger D, et al. Endotoxemia and acute- phase proteins in major abdominal surgery. Am J Surg 2001; 181:36–43
Berger D, Bölke E, Huegel H, Deidelmann M, Hannekum A, Beger HG. New aspects concerning the regulation of the post-operative acute phase reaction during cardiac surgery. Clin Chim Acta 1995; 239:121–30
Berger D, Otto S, Schmidt UM, Bölke E, Seidelmann M, Beger HG. Determination of endotoxin-neutralizing capacity of plasma in post-surgical patients. Eur Surg Res 1996; 28:130–9
Hiki N, Berger D, Buttenschoen K, Boelke E, Seidelmann M, Strecker W, Kinzl L, Beger HG. Endotoxemia and specific antibody behaviour against different endotoxins following multiple injuries. J Trauma 1995; 38:794–801
Bennet-Guerrero E, Panah MH, Barclay GB, Bodian CA, Winfree WJ, Andres LA, Reich DL, Mythen MG. Decreased endotoxin immunity is associated with greater mortality and/or prologed hospitalization after surgery. Anesthesiology 2001; 94:992–8
Snyderman CH, Kachman K, Molseed L, Wagner R, D’Amico F, Bumpous J, Rueger R. Reduced postoperative infections with an immune-enhancing nutritional supplement. Laryngoscope 1999; 109:915–21
Braga M, Gianotti L, Vignali A, Carlo VD. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic response and outcome after colorectal resection for cancer. Surgery 2002; 132:805–14
Braga M, Gianotti L, Nespoli L, Radealli G, Di Carlo V. Nutritional approach in malnourished surgical patients: a prospective randomized study. Arch Surg 2002; 137:174–80
Zulfikaroglu B, Zulfikaroglu E, Ozmen MM, Ozalp N, Berkem R, Erdogan S, Besler HT, Koc M, Korkmaz A. The effect of immunonutrition on bacterial translocation, and intestinal villus atrophy in experimental obstructive jaundice. Clin Nutr 2003; 22:277–81
Tracey KJ, Vlassara H, Cerami A. Cachetin/tumour necrosis factor. Lancet 1989; 8527:1122–6
Hesse DG, Tracey KJ, Fong Y, Manogue KR, Palladino MA Jr, Cerami A, Shires GT, Lowry SF. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet 1988; 166:147–153
Fong Y, Moldawar LL, Marano M. Cachetin/TNF or IL-1 alpha induces cachexia with redistribution of body proteins. Am J Physiol 1989; 256:659–65
Dinarello CA, Cannon JG, Wolff SM. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin-1. J Exp Med 1986; 163:1433–50
Calandra T, Baumgartner JD, Grau GE. Prognostic value of tumor necrosis factor-cachectin, interleukin-1, interferon-alpha and interferon-gamma in the serum of patients with septic shock. J Infect Dis 1990; 161:982–7
Waage A, Halstensen A, Spevik T. Association between tumor necrosis factor in serum and total outcome in patients with meningococcal disease. Lancet 1987; 8527:355–7
Senkal M, Kemen M, Homann HH, Eickhoff U, Baier J, Zumtobel V. Modulation of postoperative immune response by enteral nutrition with a diet enriched with arginine, RNA, and omega-3 fatty acids in patients with upper gastrointestinal cancer. Eur J Surg 1995; 161:115–22
Gianotti L, Braga M, Fortis C, Soldini L, Vignali A, Colombo S, Radealli G, Di Carlo V. A prospective, randomized clinical trial on perioperative feeding with an arginine-, omega-3 fatty acid-, and RNA-enriched enteral diet: effect on host response and nutritional status. JPEN 1999; 6:314–20
Helfgott DC, May LT, Sthoeger Z. Bacterial lipopolysaccharide (endotoxin) enhances expression and secretion of β2 interferon by human fibroblasts. J Exp Med 1987; 166:1300–9
Delogu G, Casula MA, Mancini P, Tellan G, Signore L. Serum neopterin and soluble interleukin-2 receptor for prediction of a shock state in gram-negative sepsis. J Crit Care 1995: 10:64–71
Strohmaier W, Redl H, Schlag G, Inthorn D. D-erythro-neopterin plasma levels in intensive care patients with and without septic complications. Crit Care Med 1987; 15:757–60
ACKNOWLEDGMENT
Supported in part by a Novartis Consumer Health Research Grant, Novartis Consumer Health Switzerland, Montbijoustrasse 188, CH-300, Berne, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giger, U., Büchler, M., Farhadi, J. et al. Preoperative Immunonutrition Suppresses Perioperative Inflammatory Response in Patients with Major Abdominal Surgery—A Randomized Controlled Pilot Study. Ann Surg Oncol 14, 2798–2806 (2007). https://doi.org/10.1245/s10434-007-9407-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-007-9407-7