Abstract
Background
Although changes in lipid profile have been documented in gastrointestinal cancers, the specific relationship between serum lipid levels and lymph node (N) stages in gastric cancer remains uncertain.
Methods
Preoperative serum lipid concentrations were retrospectively examined in 501 patients who underwent curative resection for primary gastric cancer. Univariate and multivariate analyses were carried out to investigate the association of serum lipid levels with nodal stages.
Results
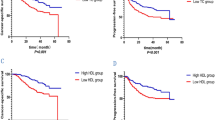
The serum high-density lipoprotein cholesterol (HDL-C) levels in gastric cancer patients correlated well with N stages. Cases with low HDL-C (<40 mg/dl) presented more advanced pN2–3 disease (57.6%, 83/144) than those with normal HDL-C (36.4%, 130/357; P<0.05). The observed elevation of the TC/HDL-C ratio for patients with pN2–3 disease was statistically significant when compared with that for patients with pN0–1 disease (mean values 3.57 vs. 3.31, P < 0.05). By receiver operating characteristic analysis, the combination of serum HDL-C concentration and TC/HDL-C ratio led to a sensitivity of 70.0% and a specificity of 51.0% in predicting stage pN2–3. A logistic regression model revealed that both low HDL-C (<40 mg/dl) and high TC/HDL-C ratio (≥3.32) had an independent association with advanced pN2–3 stages. When patients’ lipid levels were stratified by means of histological differentiation, the significant correlation of serum HDL-C levels with nodal stages was only detected in differentiated gastric cancer.
Conclusions
For gastric cancer patients, preoperative low serum HDL-C concentration or high TC/HDL-C ratio might be a potential biomarker of advanced pN2–3 stages, especially for those with the histologically differentiated type.


Similar content being viewed by others
References
Boring CC, Squires TS, Tong T, Montgomery S. Cancer statistics, 1994. CA Cancer J Clin 1994; 44(1):7–26
Sun XD, Mu R, Zhou YS, Dai XD, Zhang SW, Huangfu XM, Sun J, Li LD, Lu FZ, Qiao YL. Analysis of mortality rate of stomach cancer and its trend in twenty years in China. Zhonghua Zhong Liu Za Zhi 2004; 26(1):4–9. (in Chinese)
Meyer HJ, Jahne J. Lymph node dissection for gastric cancer. Semin Surg Oncol 1999; 17(2):117–124
Roukos DH. Current status and future perspectives in gastric cancer management. Cancer Treat Rev 2000; 26(4):243–255
Kodama Y, Sugimachi K, Soejima K, Matsusaka T, Inokuchi K. Evaluation of extensive lymph node dissection for carcinoma of the stomach. World J Surg 1981; 5(2):241–248
Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg 1987; 11(4):418–425
Roukos DH. Therapeutic value of D2-resection in gastric cancer evaluated with a new concept. UICC Consensus Conference. In: Kim JP, Min JS, Mok YJ (eds). Proceedings of the 3rd International Gastric Cancer Congress, Seoul 1999. Monduzzi Editore, Bologna (Italy), 1999:29–33
Bunt AM, Hermans J, Smit VT, van de Velde CJ, Fleuren GJ, Bruijn JA. Surgical/pathologic-stage migration confounds comparisons of gastric cancer survival rates between Japan and Western countries. J Clin Oncol 1995; 13(1):19–25
Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H, Gouma DJ, van Lanschot JJ, Taat CW, de Graaf PW, von Meyenfeldt MF, Tilanus H; Dutch Gastric Cancer Group. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999; 340(12):908–914
Alexopoulos CG, Blatsios B, Avgerinos A. Serum lipids and lipoprotein disorders in cancer patients. Cancer 1987; 60(12):3065–3070
Fiorenza AM, Branchi A, Sommariva D. Serum lipoprotein profile in patients with cancer. A comparison with non-cancer subjects. Int J Clin Lab Res 2000; 30(3):141–145
Kitayama J, Hatano K, Kaisaki S, Suzuki H, Fujii S, Nagawa H. Hyperlipidaemia is positively correlated with lymph node metastasis in men with early gastric cancer. Br J Surg 2004; 91(2):191–198
Zielinski CC, Stuller I, Rausch P, Muller C. Increased serum concentrations of cholesterol and triglycerides in the progression of breast cancer. J Cancer Res Clin Oncol 1988; 114(5):514–518
Gaziano JM, Hennekens CH. Dietary fat and risk of prostate cancer. J Natl Cancer Inst 1995; 87(19):1427–1428
Howe GR, Aronson KJ, Benito E, Castelleto R, Cornee J, Duffy S, Gallagher RP, Iscovich JM, Deng-ao J, Kaaks R, Kune GA, Kune S, Shu Z, et al. The relationship between dietary fat intake and risk of colorectal cancer: evidence from the combined analysis of 13 case-control studies. Cancer Causes Control 1997; 8(2):215–228
Sobin LH, Wittekind C. International Union Against Cancer (UICC). TNM Classification of Malignant Tumors, 5th edn. Wiley, New York. 1997:59–62
Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer 1998; 1(1):10–24
Adachi Y, Mori M, Kido A, Shimono R, Maehara Y, Sugimachi K. A clinicopathologic study of mucinous gastric carcinoma. Cancer 1992; 69(4):866–871
Bayerdorffer E, Mannes GA, Richter WO, Ochsenkuhn T, Seeholzer G, Kopcke W, Wiebecke B, Paumgartner G. Decreased high-density lipoprotein cholesterol and increased low-density cholesterol levels in patients with colorectal adenomas. Ann Intern Med 1993; 118(7):481–487
National Institutes of Health, National Heart, Lung and Blood Institute. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106(25):3143–3421
Michalaki V, Koutroulis G, Syrigos K, Piperi C, Kalofoutis A. Evaluation of serum lipids and high-density lipoprotein subfractions (HDL2,HDL3) in postmenopausal patients with breast cancer. Mol Cell Biochem 2005; 268(1–2):19–24
Chan AO, Chu KM, Lam SK, Cheung KL, Law S, Kwok KF, Wong WM, Yuen MF, Wong BC. Early prediction of tumor recurrence after curative resection of gastric carcinoma by measuring soluble E-cadherin. Cancer 2005; 104(4):740–746
Ishii M, Gama H, Chida N, et al. Simultaneous measurements of serum alpha-fetoprotein and protein induced by vitamin K absence for detecting hepatocellular carcinoma. South Tohoku District Study Group. Am J Gastroenterol 2000; 95(4):1036–1040
Eisenberg S. High density lipoprotein metabolism. J Lipid Res 1984; 25(10):1017–1058
Daerr WH, Gianturco SH, Patsch JR, Smith LC, Gotto AM Jr. Stimulation and suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase in normal human fibroblasts by high density lipoprotein subclasses. Biochim Biophys Acta 1980; 619(2):287–301
Daniels RJ, Guertler LS, Parker TS, Steinberg D. Studies on the rate of efflux of cholesterol from cultured human skin fibroblasts. J Biol Chem 1981; 256(10):4978–4983
Oram JF, Johnson CJ, Brown TA. Interaction of high density lipoprotein with its receptor on cultured fibroblasts and macrophages. J Biol Chem 1987; 262(5):2405–2410
Dessi S, Batetta B, Laconi E, Ennas C, Pani P. Hepatic cholesterol in lead nitrate induced liver hyperplasia. Chem Biol Interact 1984; 48(3):271–279
Dessi S, Batetta B, Carrucciu A, Pulisci D, Laconi S, Fadda AM, Anchisi C, Pani P. Variations of serum lipoproteins during cell proliferation induced by lead nitrate. Exp Mol Pathol 1989; 51(2):97–102
Dessi S, Batetta B, Anchisi C, Pani P, Costelli P, Tessitore L, Baccino FM. Cholesterol metabolism during the growth of a rat ascites hepatoma (Yoshida AH-130). Br J Cancer 1992; 66(5):787–793
Adachi Y, Mori M, Enjoji M, Sugimachi K. Microvascular architecture of early gastric carcinoma. Microvascular-histopathologic correlates. Cancer 1993; 72(1):32–36
Ziegler K, Sanft C, Zimmer T, et al. Comparison of computed tomography, endosonography, and intraoperative assessment in TN staging of gastric carcinoma. Gut 1993; 34(5):604–610
Habermann CR, Weiss F, Riecken R, et al. Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology 2004; 230(2):465–471
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation of China (no. 30471697 and no. 30672072), Natural Science Foundation of Zhejiang, China (no. Z204033) and Foundation of Science and Technology Department of Zhejiang Province, China (no. 2006C23G2010216). *The first two authors contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
The abbreviation of pT and pN denotes pathologically confirmed tumor-nodes.
Rights and permissions
About this article
Cite this article
Guo, E., Chen, L., Xie, Q. et al. Serum HDL-C as a Potential Biomarker for Nodal Stages in Gastric Cancer. Ann Surg Oncol 14, 2528–2534 (2007). https://doi.org/10.1245/s10434-007-9401-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-007-9401-0