Abstract
Background
Peritoneal cytology is an important prognostic factor of gastric cancer. However, peritoneal cytology requires great skill, which may explain its low prevalence. A reverse transcriptase–polymerase chain reaction–based assay with multiple marker genes or immunocytochemistry was assessed as an alternative method of gathering the same kind of data as cytology.
Methods
Peritoneal washings from 179 patients with gastric cancer were analyzed by multiplex reverse transcriptase–polymerase chain reaction with 10 marker genes and subsequent hybridization to a customized oligo-nucleotide array. Results with this assay were either validated as a prognostic factor or confirmed by demonstrating the presence of cancer cells by immunocytochemical cytology.
Results
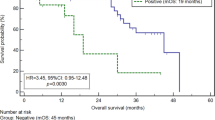
Only 1 (2.2%) of 44 disease-free cases was shown to be positive by the microarray assay, whereas 13 (93%) of 14 conventional cytology–positive cases were found to be positive. This assay further detected approximately one-third of cytology-negative patients either with peritoneal recurrence (7 of 20, 35%) or with non-peritoneal recurrence (6 of 22, 27%). A high concordance between the microarray assay and immunocytochemical cytology with five antibodies against CK20, FABP1, MUC2, TFF1, and MASPIN was confirmed. The clinical outcome of the microarray assay–positive cases was poor, as was that of the cytology-positive cases.
Conclusions
Our assay, though time-consuming and requiring special equipment, demonstrated a specificity and sensitivity equal to or better than cytology in our institutes. The minimal free peritoneal cancer cells detected by the microarray assay may provide the same clinical information as larger amounts of cancer cells for patients with gastric cancer. An anti-MASPIN antibody may be helpful in peritoneal cytology of gastric cancer.





Similar content being viewed by others
References
Ferlay J, Bray F, Pisani P, et al. Globocan 2000: Cancer Incidence, Mortality, and Prevalence Worldwide. Ver 1.0, vol 5. Lyon: IARC Press, 2001
Wu CW, Lo SS, Shen KH, et al. Incidence and factors associated with recurrence patterns after intended curative surgery for gastric cancer. World J Surg 2003;27:153–8
Bonenkamp JJ, Songun I, Hermans J, et al. Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg 1996;83:672–4
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 2nd English ed. Gastric Cancer 1998;1:10–24
Onate-Ocana LF, Gallardo-Rincon D, Aiello-Crocifoglio V, et al. The role of pretherapeutic laparoscopy in the selection of treatment for patients with gastric carcinoma: a proposal for a laparoscopic staging system. Ann Surg Oncol 2001;8:624–31
Yano M, Yasuda T, Fujiwara Y, et al. Preoperative intraperitoneal chemotherapy for patients with serosa-infiltrating gastric cancer. J Surg Oncol 2004;88:39–43
Kodera Y, Nakanishi H, Ito S, et al. Quantitative detection of disseminated free cancer cells in peritoneal washes with real-time reverse transcriptase–polymerase chain reaction. Ann Surg 2002;235:499–506
Fujimura T, Ohta T, Kitagawa H, et al. Trypsinogen expression and early detection for peritoneal dissemination in gastric cancer. J Surg Oncol 1998;69:71–5
Benevolo M, Mottolese M, Cosimelli M, et al. Diagnostic and prognostic value of peritoneal immunocytology in gastric cancer. J Clin Oncol 1998;16:3406–11
Nagao K, Hisatomi H, Hirata H, et al. Expression of molecular marker genes in various types of normal tissue: implication for detection of micrometastases. Int J Mol Med 2002;10:307–10
Kubota K, Nakanishi H, Hiki N, et al. Quantitative detection of micrometastases in the lymph nodes of gastric cancer patients with real-time RT-PCR: a comparative study with immunohistochemistry. Int J Cancer 2003;105:136–43
Mori K, Aoyagi K, Ueda T, et al. Highly specific marker genes for detecting minimal gastric cancer cells in cytology negative peritoneal washings. Biochem Biophys Res Commun 2004;313:931–7
Taback B, Chan AD, Kuo CT, et al. Detection of occult metastatic breast cancer cells in blood by a multimolecular marker assay: correlation with clinical stage of disease. Cancer Res 2001;61:8845–50
Okamoto T, Suzuki T, Yamamoto N. Microarray fabricaton with covalent attachment of DNA using Bubble Jet technology. Nat Biotech 2000;18:438–41
Ushijima T, Sasako M. Focus on gastric cancer. Cancer Cell 2004;5:121–5
Ishigami S, Natsugoe S, Tokuda K, et al. Clinical impact of micrometastasis of the lymph node in gastric cancer. Am Surg 2003;69:573–7
Mattano LA Jr, Moss TJ, Emerson SG. Sensitive detection of rare circulating neuroblastoma cells by the reverse transcriptase-polymerase chain reaction. Cancer Res 1992;52:4701–5
Sakakura C, Takemura M, Hagiwara A, et al. Overexpression of dopa decarboxylase in peritoneal dissemination of gastric cancer and its potential as a novel marker for the detection of peritoneal micrometastases with real-time RT-PCR. Br J Cancer 2004;90:665–71
Wang ZN, Xu HM, Jiang L, et al. Expression of survivin in primary and metastatic gastric cancer cells obtained by laser capture microdissection. World J Gastroenterol 2004;10:3094–8
Harstrick A, Klaassen U, Eberhardt W, et al. Role of chemotherapy against micrometastatic cancer. Cancer Metastasis Rev 1999;18:151–72
Day RS, Shackney SE, Peters WP. The analysis of relapse-free survival curves: implications for evaluating intensive systemic adjuvant treatment regimens for breast cancer. Br J Cancer 2005;92:47–54
Acknowledgments
Supported in part by a grant from the program for the Promotion of Fundamental Studies in Health Sciences of the National Institute of Biochemical Innovation (NiBio); and by a Grant-in-Aid for the Third Comprehensive 10-Year Strategy for Cancer Control and for Cancer Research (15-5 and 16-15) from the Ministry of Health, Labour and Welfare of Japan. K.M. was a recipient of Research Resident Fellowships from the Foundation for Promotion of Cancer Research. We thank Mr. Kiyoaki Nomoto for his assistance in immunocytochemistry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mori, K., Suzuki, T., Uozaki, H. et al. Detection of Minimal Gastric Cancer Cells in Peritoneal Washings by Focused Microarray Analysis with Multiple Markers: Clinical Implications. Ann Surg Oncol 14, 1694–1702 (2007). https://doi.org/10.1245/s10434-006-9321-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-006-9321-4