Abstract
Background: We analyzed the histological features and DNA flow cytometric results in 34 patients with pheochromocytoma and paragangliomas and attempted correlation with the biological behavior for determination of the malignant potential of these tumors.
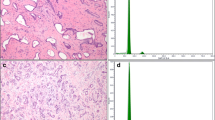
Methods: DNA analysis was done on a FACSort flow cytometer using paraffin-embedded tissues. Histopathological analysis was performed using parameters, i.e., cell size (large, medium, and small), cell size variation, mitotic rate, nuclear pleomorphism, golden yellow to brown pigment in the tumor, necrosis, and venous invasion.
Results: Six tumors had high (>5/10HPF) mitotic rate while venous invasion was seen in three tumors. Fifty percent (18/34) of patients had aneuploid tumors, and 68% (23/34) of patients had high (>10%) S-phase fraction tumors. Aneuploidy correlated with >5/10HPF mitotic rate (P < .05) and diploidy with golden yellow to brown pigment (P < .01). The patients with aneuploid tumor had a worse prognosis than patients with diploid tumors (P = .004). No such difference was observed with low and high S-phase fractions (P = .748), presence and absence of venous invasion (P = .927), and mitotic rate (P = .159). Nuclear pleomorphism and necrosis were not significant factors in prognosis.
Conclusions:Flow cytometric DNA analysis of paragangliomas and pheochromocytomas correlated with biological behavior in the patients with regard to metastasis and overall survival in the patients.
Similar content being viewed by others
REFERENCES
Schlumberger M, Gicquel C, Lumbroso J, et al. Malignant pheochromocytoma: clinical, biological, histologic and therapeutic data in a series of 20 patients with distant metastases. J Endocrinol Invest 1992;15:631–42.
Proye C, Vix M, Goropoulos A, Kerlo P, Lecomte-Houcke M. High incidence of malignant pheochromocytoma in a surgical unit. 26 cases out of 100 patients operated from 1971 to 1991. J Endocrinol Invest 1992;15:651–63.
Heinbecker P, O’Neal LW, Ackerman LV. Functioning and nonfunctioning adrenal cortical tumors. Surg Gynecol Obstract 1957;105:21–33.
Lack EE. Afip Atlas of Tumor Pathology: Tumors of the Adrenal Gland and Extra Adrenal Paraganglia. Washington, DC: American Registry of Pathology, 1997.
Olson JL, Salyer WR. Mediastinal paragangliomas (aortic body tumor): a report of four cases and a review of the literature. Cancer 1978;41:2405–12.
Lack EE, Cubilla AL, Woodruff JM, Lieberman PH. Extra-adrenal paragangliomas of the retroperitoneum: a clinicopathologic study of 12 tumors. Am J Surg Pathol 1980;4:109–20.
Linnoila RI, Keiser HR, Steinberg SM, Lack EE. Histopathology of benign versus malignant sympathoadrenal paragangliomas: clinicopathologic study of 120 cases including unusual histologic features. Hum Pathol 1990;21:1168–80.
Moyer TP, Jiang NS, Tyce GM, Sheps SG. Analysis for urinary catecholamines by liquid chromatography with amperometric detection: methodology and clinical interpretation of results. Clin Chem 1979;25:256–63.
Cheung PS, Thompson NW, Dmuchowski CF, Sisson JC. Spectrum of pheochromocytoma in the 131I-MIBG era. World J Surg 1988;12:546–51.
Nakada T, Kubota Y, Sasagawa I, Yagisawa T, Watanabe M, Ishigooka M. Remarkably suppressed manganese superoxide dismutase activity in malignant pheochromocytoma. J Urol 1995;153:1787–90.
Kubota Y, Nakada T, Sasagawa I, Yanai H, Itoh K. Elevated levels of telomerase activity in malignant pheochromocytoma. Cancer 1998;82:176–9.
Hirano Y, Fujita K, Suzuki K, Ushiyama T, Ohtawara Y, Tsuda F. Telomerase activity as an indicator of potentially malignant adrenal tumors. Cancer 1998;83:772–6.
Hosaka Y, Rainwater LM, Grant CS, Farrow GM, van Heerden JA, Lieber MM. Pheochromocytoma: nuclear deoxyribonucleic acid patterns studied by flow cytometry. Surgery 1986;100:1003–10.
Gonzalez-Campora R, Diaz Cano S, Lerma-Puertas E, et al. Paragangliomas. Static cytometric studies of nuclear DNA patterns. Cancer 1993;71:820–4.
Nativ O, Grant CS, Sheps SG, et al. The clinical significance of nuclear DNA ploidy pattern in 184 patients with pheochromocytoma. Cancer 1992;69:2683–7.
Hedley DW, Friedlander ML, Taylor IW, Rugg CA, Musgrove EA. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem 1983;31:1333–5.
Norusis MJ. SPSS for Windows, Advanced Statistics, Release 6.0. Chicago, IL: SPSS Inc., 1993.
Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81.
Modlin IM, Farndon JR, Shepherd A, et al. Phaeochromocytomas in 72 patients: clinical and diagnostic features, treatment and long term results. Br J Surg 1979;66:456–65.
Remine WH, Chong GC, Van Heerden JA, Sheps SG, Harrison EG Jr. Current management of pheochromocytoma. Ann Surg 1974;179:740–8.
Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg 1984;8:163–9.
Klein FA, Kay S, Ratliff JE, White FK, Newsome HH. Flow cytometric determinations of ploidy and proliferation patterns of adrenal neoplasms: an adjunct to histological classification. J Urol 1985;134:862–6.
Lewis PD. A cytophotometric study of benign and malignant phaeochromocytomas. Virchows Arch B Cell Pathol 1971;9:371–6.
Amberson JB, Vaughan ED Jr, Gray GF, Naus GJ. Flow cytometric analysis of nuclear DNA from adrenocortical neoplasms. A retrospective study using paraffin-embedded tissue. Cancer 1987;59:2091–5.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, M.J., Karelia, N.H., Patel, S.M. et al. Flow Cytometric DNA Analysis for Determination of Malignant Potential in Adrenal Pheochromocytoma or Paraganglioma: An Indian Experience. Ann Surg Oncol 10, 426–431 (2003). https://doi.org/10.1245/ASO.2003.04.007
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1245/ASO.2003.04.007