Abstract
Background
Patients with borderline (BR) or locally advanced (LA) pancreatic adenocarcinoma (PAC) are often treated with induction FOLFIRINOX (FLX). However, the role of additional preoperative chemoradiotherapy (CRT) is controversial. The aim of this study is to evaluate its impact in patients who underwent resection after induction FLX.
Patients and Methods
Retrospective analysis of prospective consecutive surgical BR or LA PAC patients after induction FLX in 23 French centers between November 2010 and December 2015, treated with or without preoperative additional CRT (FLX vs FLX + CRT groups).
Results
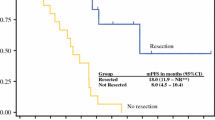
Two hundred three patients were included (106 BR, 97 LA PAC). Median number of FLX cycles was 6 (range 1–30); 50% (n = 102) of patients received additional CRT. Median duration between diagnosis and surgery was 5.4 and 8.7 months (P = 0.001) in the FLX and FLX + CRT group, respectively. The 90-day mortality, major complications, and pancreatic fistula rates were 4.4%, 17.7%, and 5.4%, respectively. After 45.1 months follow-up, overall survival (OS) and disease-free survival were 45.4 months and 16.2 months, respectively. Patients with additional CRT had higher R0 resection rate (89.2% vs 76.3%; P = 0.017), ypN0 rate (76.2% vs 48.5%; P < 0.001), and higher rate of pathologic major response (33.3% vs 12.9%; P = 0.001). In the FLX + CRT group, patients had lower rate of locoregional relapse (28.3% vs 50.7%; P = 0.004). Patients with additional CRT had longer OS than those receiving FLX alone (57.8 vs 35.5 months; P = 0.007).
Conclusions
Pathological results and survival data argue for interest in additional CRT. Prospective studies on an intention-to-treat basis are needed to confirm these results.


Similar content being viewed by others
References
Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma. J Natl Compr Cancer Netw JNCCN. 2017;15(8):1028–61.
Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18(1):2–11.
Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–13.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Christians KK, Tsai S, Mahmoud A, et al. Neoadjuvant FOLFIRINOX for borderline resectable pancreas cancer: a new treatment paradigm? Oncologist. 2014;19(3):266–74.
Faris JE, Blaszkowsky LS, McDermott S, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. 2013;18(5):543–8.
Marthey L, Sa-Cunha A, Blanc JF, et al. FOLFIRINOX for locally advanced pancreatic adenocarcinoma: results of an AGEO multicenter prospective observational cohort. Ann Surg Oncol. 2015;22(1):295–301.
Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol. 2015;22(4):1153–9.
Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48(8):1705–10.
Hazel JJ, Thirlwell MP, Huggins M, Maksymiuk A, MacFarlane JK. Multi-drug chemotherapy with and without radiation for carcinoma of the stomach and pancreas: a prospective randomized trial. J Can Assoc Radiol. 1981;32(3):164–5.
Klaassen DJ, MacIntyre JM, Catton GE, Engstrom PF, Moertel CG. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil—an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3(3):373–8.
Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol. 2008;19(9):1592–9.
Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst. 1988;80(10):751–5.
Loehrer PJ, Sr., Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29(31):4105–12.
Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315(17):1844–53.
Pietrasz D, Marthey L, Wagner M, et al. Pathologic major response after FOLFIRINOX is prognostic for patients secondary resected for borderline or locally advanced pancreatic adenocarcinoma: an AGEO-FRENCH, prospective, multicentric cohort. Ann Surg Oncol. 2015;22 Suppl 3:1196–205.
Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17(6):801–10.
Huguet F, Goodman KA, Azria D, Racadot S, Abrams RA. Radiotherapy technical considerations in the management of locally advanced pancreatic cancer: American–French consensus recommendations. Int J Radiat Oncol Biol Phys. 2012;83(5):1355–64.
Evans DB, George B, Tsai S. Non-metastatic pancreatic cancer: resectable, borderline resectable, and locally advanced-definitions of increasing importance for the optimal delivery of multimodality therapy. Ann Surg Oncol. 2015;22(11):3409–13.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584–91.
Edge SB, Compton CC. The AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4.
Staley CA, Cleary KR, Abbruzzese JL, et al. The need for standardized pathologic staging of pancreaticoduodenectomy specimens. Pancreas. 1996;12(4):373–80.
Bonnetain F, Bonsing B, Conroy T, et al. Guidelines for time-to-event end-point definitions in trials for pancreatic cancer. Results of the DATECAN initiative (Definition for the Assessment of Time-to-event End-points in CANcer trials). Eur J Cancer. 2014;50(17):2983–93.
Schlieman MG, Ho HS, Bold RJ. Utility of tumor markers in determining resectability of pancreatic cancer. Arch Surg. 2003;138(9):951–6 (discussion 955–956).
Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261(1):12–7.
Sadot E, Doussot A, O’Reilly EM, et al. FOLFIRINOX induction therapy for stage 3 pancreatic adenocarcinoma. Ann Surg Oncol. 2015;22(11):3512–21.
He J, Blair AB, Groot VP, et al. Is a pathological complete response following neoadjuvant chemoradiation associated with prolonged survival in patients with pancreatic cancer? Ann Surg. 2018;268(1):1–8.
Acknowledgment
Daniel Pietrasz, Jean-Baptiste Bachet and Antonio Sa Cunha have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have nothing to disclose.
Clinical Investigators Who Collected Data, Provided, and Cared for Study Patients
Aparicio Thomas, MD, PhD (Avicenne Hospital, Bobigny, France); Berger Anne, MD, PhD (Georges Pompidou European Hospital, Paris, France); Bourdariat Raphaël, MD (Jean Mermoz Hospital, Lyon, France); Blanc Jean-Frédéric (Hôpital Haut-Lévêque, CHU Bordeaux, Pessac, France); Chiche Laurence, MD, PhD (Bordeaux South Hospital, Bordeaux, France); Colombo Pierre Emmanuel, MD, PhD (Institut Régional du Cancer ICM, Montpellier, France); Dousset Bertrand, MD, PhD (Cochin Hospital, Paris, France); Drubay Vincent, MD (CHU Lille, Lille, France); Francois Eric, MD (Nice Hospital, Nice, France); Gilabert Marine, MD, PhD (Institut Paoli Calmette, Marseille, France); Hammel Pascal, MD, PhD (Beaujon Hospital, Clichy, France); Lecaille Cédric, MD (Bordeaux North Hospital, Bordeaux, France); Malka David (Gustave Roussy, Villejuif, France); Manfredi Sylvain, MD (Rennes Hospital, Rennes, France); Marthey Lysiane (Kremlin Bicêtre Hospital, Le Kremlin Bicêtre, France); Meunier Bernard, MD, PhD (Rennes Hospital, Rennes, France); Morere François (Paul Brousse Hospital, Villejuif France); Paye François, MD, PhD (Saint-Antoine Hospital, Paris, France); Penna Christophe (Kremlin Bicêtre Hospital, Kremlin Bicêtre, France); Pezet Denis, MD, PhD (CHU Estaing, Clermont Ferrand, France); Piessen Guillaume (CHU Lille, Lille, France); Pittau Gabriella, MD (Paul Brousse Hospiral, Villejuif France); Pointet Anne Laure, MD (Georges Pompidou European Hospital, Paris, France); Schwarz Lilian, MD (Rouen Hospital, Rouen, France); Smith Denis (Bordeaux Saint-André Hospital, Bordeaux, France); Soularue Emilie, MD (Kremlin Bicêtre Hospital, Kremlin Bicêtre, France).
Additional information
The AGEO and FRENCH associations supported this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pietrasz, D., Turrini, O., Vendrely, V. et al. How Does Chemoradiotherapy Following Induction FOLFIRINOX Improve the Results in Resected Borderline or Locally Advanced Pancreatic Adenocarcinoma? An AGEO-FRENCH Multicentric Cohort. Ann Surg Oncol 26, 109–117 (2019). https://doi.org/10.1245/s10434-018-6931-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6931-6