Abstract
Background
Cancer stem-like cells (CSCs) in colorectal cancers (CRC) may account for the failure of treatments because they are resistant to many current anticancer therapies. Salinomycin, a potassium ionophore, was recently identified as a selective inhibitor of breast CSCs.
Methods
The human CRC cell lines HT29 and SW480 were treated with salinomycin and oxaliplatin. Cell viability was determined with cell counting kit 8. Fraction of CD133+ cell subpopulations was assessed by Flow Cytometric analysis. Clonogenecity and migration were determined with soft agar and Boyden chamber assays. Molecular changes were assessed by immunofluorescence staining, RT-PCR, and Western blot analysis.
Results
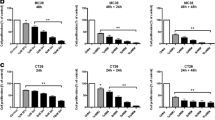
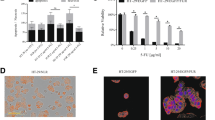
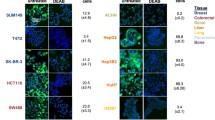
We report that salinomycin reduces the proportion of CD133+ subpopulations in human CRC HT29 and SW480 cells. Furthermore, salinomycin treatment decreases colony-forming ability and cell motility in HT29 cells. Moreover, salinomycin downregulates the expression of vimentin and induces the E-cadherin expression in HT29 cells.
Conclusions
This study demonstrates the ability of salinomycin to selectively target “CD133+” cell subpopulations and decrease the malignant traits in colorectal cancer lines.





Similar content being viewed by others
References
O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–10.
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–5.
Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104(24):10158–63.
Ieta K, Tanaka F, Haraguchi N, Kita Y, Sakashita H, Mimori K, Matsumoto T, Inoue H, Kuwano H, Mori M. Biological and genetic characteristics of tumor-initiating cells in colon cancer. Ann Surg Oncol. 2008;15(2):638–48.
Haraguchi N, Ohkuma M, Sakashita H, Matsuzaki S, Tanaka F, Mimori K, Kamohara Y, Inoue H, Mori M. CD133+ CD44+ population efficiently enriches colon cancer initiating cells. Ann Surg Oncol. 2008;15(10):2927–33.
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–60.
Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–84.
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780–3.
Diehn M, Clarke MF. Cancer stem cells and radiotherapy: new insights into tumor radioresistance. J Natl Cancer Inst. 2006;98(24):1755–7.
Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26(17):2839–45.
Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1(4):389–402.
Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–59.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15.
Yang AD, Fan F, Camp ER, van Buren G, Liu W, Somcio R, Gray MJ, Cheng H, Hoff PM, Ellis LM. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 2006;12(14 Pt 1):4147–53.
Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11(12):1487–95.
Neuzil J, Stantic M, Zobalova R, Chladova J, Wang X, Prochazka L, Dong L, Andera L, Ralph SJ. Tumour-initiating cells vs. cancer ‘stem’ cells and CD133: what’s in the name? Biochem Biophys Res Commun. 2007;355(4):855–9.
Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68(10):3645–54.
Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–73.
Ong CW, Kim LG, Kong HH, Low LY, Iacopetta B, Soong R, Salto-Tellez M. CD133 expression predicts for non-response to chemotherapy in colorectal cancer. Mod Pathol. 23(3):450–7.
Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Ioue Y, Miki C, Kusunoki M. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol. 2009;16(12):3488–98.
Artells R, Moreno I, Diaz T, Martinez F, Gel B, Navarro A, Ibeas R, Moreno J, Monzo M. Tumour CD133 mRNA expression and clinical outcome in surgically resected colorectal cancer patients. Eur J Cancer. 2010;46(3):642–9.
Corbeil D, Roper K, Hellwig A, Tavian M, Miraglia S, Watt SM, Simmons PJ, Peault B, Buck DW, Huttner WB. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 2000;275(8):5512–20.
Giebel B, Corbeil D, Beckmann J, Hohn J, Freund D, Giesen K, Fischer J, Kogler G, Wernet P. Segregation of lipid raft markers including CD133 in polarized human hematopoietic stem and progenitor cells. Blood. 2004;104(8):2332–8.
Elsaba TM, Martinez-Pomares L, Robins AR, Crook S, Seth R, Jackson D, McCart A, Silver AR, Tomlinson IP, Ilyas M. The stem cell marker CD133 associates with enhanced colony formation and cell motility in colorectal cancer. PLoS One. 2010;5(5):e10714.
Rappa G, Fodstad O, Lorico A. The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells. 2008;26(12):3008–17.
Fuchs D, Heinold A, Opelz G, Daniel V, Naujokat C. Salinomycin induces apoptosis and overcomes apoptosis resistance in human cancer cells. Biochem Biophys Res Commun. 2009;390(3):743–9.
Riccioni R, Dupuis ML, Bernabei M, Petrucci E, Pasquini L, Mariani G, Cianfriglia M, Testa U. The cancer stem cell selective inhibitor salinomycin is a p-glycoprotein inhibitor. Blood Cells Mol Dis. 2010;45(1):86–92.
Fuchs D, Daniel V, Sadeghi M, Opelz G, Naujokat C. Salinomycin overcomes ABC transporter-mediated multidrug and apoptosis resistance in human leukemia stem cell-like KG-1a cells. Biochem Biophys Res Commun. 2010;394(4):1098–104.
Yvore P, Raynaud JP, Conan L, Naciri M. Evaluation of the efficacy of salinomycin in the control of coccidiosis in chicks. Poult Sci. 1980;59(11):2412–6.
Callaway TR, Edrington TS, Rychlik JL, Genovese KJ, Poole TL, Jung YS, Bischoff KM, Anderson RC, Nisbet DJ. Ionophores: their use as ruminant growth promotants and impact on food safety. Curr Issues Intest Microbiol. 2003;4(2):43–51.
Li Y, Fang J, Wu S, Ma K, Li H, Yan X, Dong F. Identification and quantification of salinomycin in intoxicated human plasma by liquid chromatography-electrospray tandem mass spectrometry. Anal Bioanal Chem. 2010;398(2):955–61.
Story P, Doube A. A case of human poisoning by salinomycin, an agricultural antibiotic. N Z Med J. 2004;117(1190):U799.
Kojima M, Ishii G, Atsumi N, Nishizawa Y, Saito N, Ochiai A. CD133 expression in rectal cancer after preoperative chemoradiotherapy. Cancer Sci. 2010;101(4):906–12.
Lazebnik Y. What are the hallmarks of cancer? Nat Rev Cancer. 2010;10(4):232–3.
Weinberg RA. The rational treatment of cancer. In: Zayatz E, editor. The biology of cancer. New York: Garland Science; 2007. p. 787–94.
Acknowledgment
This study was supported by the National Natural Science Foundation (No. 30901424) and the Leading Medical Talent Foundation of Shanghai Municipality (No. LJ06038). We thank the members of Bing-Ya Liu’s laboratory for helpful comments and discussions, Dr. Zhi-Qiang Chen for reagents, and Xiao-Shuang Yan for technical help.
Disclosure
This study was supported by the National Natural Science Foundation (No. 30901424) and the Leading Medical Talent Foundation of Shanghai Municipality (No. LJ06038). The authors declared no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tao-Tao Dong and Hou-Min Zhou contributed equally to this work.
Rights and permissions
About this article
Cite this article
Dong, TT., Zhou, HM., Wang, LL. et al. Salinomycin Selectively Targets ‘CD133+’ Cell Subpopulations and Decreases Malignant Traits in Colorectal Cancer Lines. Ann Surg Oncol 18, 1797–1804 (2011). https://doi.org/10.1245/s10434-011-1561-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-011-1561-2