Abstract
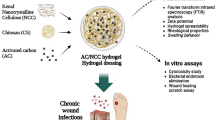
Antimicrobial peptide LL37 is a promising antibacterial candidate due to its potent antimicrobial activity with no known bacterial resistance. However, intrinsically LL37 is susceptible to degradation in wound fluids limits its effectiveness. Bacterial toxins which are released after cell lysis are found to hinder wound healing. To address these challenges, encapsulating LL37 in microspheres (MS) and loading the MS onto activated carbon (AC)-chitosan (CS) hydrogel. This advanced wound dressing not only protects LL37 from degradation but also targets bacterial toxins, aiding in the healing of chronic wound infections. First, LL37 MS and LL37-AC-CS hydrogel were prepared and characterised in terms of physicochemical properties, drug release, and peptide-polymer compatibility. Antibacterial and antibiofilm activity, bacterial toxin elimination, cell migration, and cell cytotoxicity activities were investigated. LL37-AC-CS hydrogel was effective against Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. LL37-AC-CS hydrogel bound more endotoxin than AC with CS hydrogel alone. The hydrogel also induced cell migration after 72 h and showed no cytotoxicity towards NHDF after 72 h of treatment. In conclusion, the LL37-AC-CS hydrogel was shown to be a stable, non-toxic advanced wound dressing method with enhanced antimicrobial and antitoxin activity, and it can potentially be applied to chronic wound infections to accelerate wound healing.
Graphical Abstract









Similar content being viewed by others
Data Availability
The raw data presented in this study are available on request from the corresponding author.
References
Sen CK. Human wound and its burden: Updated 2020 compendium of estimates. Adv Wound Care (New Rochelle). 2021;10:281. https://doi.org/10.1089/WOUND.2021.0026.
Sanya DRA, Onésime D, Vizzarro G, Jacquier N. Recent advances in therapeutic targets identification and development of treatment strategies towards Pseudomonas aeruginosa infections. BMC Microbiol. 2023;23:86. https://doi.org/10.1186/s12866-023-02832-x.
Paleczny J, Junka A, Brożyna M, Dydak K, Oleksy-Wawrzyniak M, Ciecholewska-Juśko D, Dziedzic E, Bartoszewicz M. The high impact of staphylococcus aureus biofilm culture medium on in vitro outcomes of antimicrobial activity of wound antiseptics and antibiotic. Pathogens. 2021;10:1387. https://doi.org/10.3390/PATHOGENS10111385/S1.
Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle). 2015;4:560. https://doi.org/10.1089/WOUND.2015.0635.
Omar A, Wright JB, Schultz G, Burrell R, Nadworny P. Microbial biofilms and chronic wounds. Microorganisms. 2017;5:9. https://doi.org/10.3390/MICROORGANISMS5010009.
James GA, Swogger E, Wolcott R, Pulcini ED, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair and Regeneration. 2008;16:37–44. https://doi.org/10.1111/J.1524-475X.2007.00321.X.
Shumba P, Shambat SM, Siemens N. The role of streptococcal and staphylococcal exotoxins and proteases in human necrotizing soft tissue infections. Toxins. 2019;11:332. https://doi.org/10.3390/TOXINS11060332.
Browne K, Chakraborty S, Chen R, Willcox MDP, Black DS, Walsh WR, Kumar N. (2020) A new era of antibiotics: The clinical potential of antimicrobial peptides. Int J Mol Sci. 2020;21(7047):21. https://doi.org/10.3390/IJMS21197047.
Lopes BS, Hanafiah A, Nachimuthu R, Muthupandian S, MdNesran ZN, Patil S. The role of antimicrobial peptides as antimicrobial and antibiofilm agents in tackling the silent pandemic of antimicrobial resistance. Molecules. 2022;27:2995. https://doi.org/10.3390/molecules27092995.
Silva JP, Appelberg R, Gama FM. Antimicrobial peptides as novel anti-tuberculosis therapeutics. Biotechnol Adv. 2016;34:924–40. https://doi.org/10.1016/j.biotechadv.2016.05.007.
Geitani R, AyoubMoubareck C, Touqui L, KaramSarkis D. Cationic antimicrobial peptides: alternatives and/or adjuvants to antibiotics active against methicillin-resistant Staphylococcus aureus and multidrug-resistant Pseudomonas aeruginosa. BMC Microbiol. 2019;19:54. https://doi.org/10.1186/s12866-019-1416-8.
Lin Z, Wu T, Wang W, Li B, Wang M, Chen L, Xia H, Zhang T. Biofunctions of antimicrobial peptide-conjugated alginate/hyaluronic acid/collagen wound dressings promote wound healing of a mixed-bacteria-infected wound. Int J Biol Macromol. 2019;140:330–42. https://doi.org/10.1016/j.ijbiomac.2019.08.087.
Dürr UHN, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides, Biochimica et Biophysica Acta (BBA). Biomembranes. 2006;1758:1408–25. https://doi.org/10.1016/j.bbamem.2006.03.030.
Yang B, Good D, Mosaiab T, Liu W, Ni G, Kaur J, Liu X, Jessop C, Yang L, Fadhil R, Yi Z, Wei MQ. Significance of LL-37 on immunomodulation and disease outcome. Biomed Res Int. 2020;2020:8349712. https://doi.org/10.1155/2020/8349712.
Rippon MG, Westgate S, Rogers AA. Implications of endotoxins in wound healing: a narrative review. J Wound Care. 2022;31:380–92. https://doi.org/10.12968/jowc.2022.31.5.380.
Osmokrovic A, Jancic I, Vunduk J, Petrovic P, Milenkovic M, Obradovic B. Achieving high antimicrobial activity: Composite alginate hydrogel beads releasing activated charcoal with an immobilized active agent. Carbohydr Polym. 2018;196:279–88. https://doi.org/10.1016/j.carbpol.2018.05.045.
Chereddy KK, Her C-H, Comune M, Moia C, Lopes A, Porporato PE, Vanacker J, Lam MC, Steinstraesser L, Sonveaux P, Zhu H, Ferreira LS, Vandermeulen G, Préat V. PLGA nanoparticles loaded with host defense peptide LL37 promote wound healing. J Contr Release. 2014;194:138–47. https://doi.org/10.1016/j.jconrel.2014.08.016.
Thoniyot P, Tan MJ, Karim AA, Young DJ, Loh XJ. Nanoparticle-hydrogel composites: concept, design, and applications of these promising, multi-functional materials. Adv Sci (Weinh). 2015;2(1–2):1400010. https://doi.org/10.1002/advs.201400010.
Puvirajesinghe TM, Zhi ZL, Craster RV, Guenneau S. Tailoring drug release rates in hydrogel-based therapeutic delivery applications using graphene oxide. J R Soc Interface. 2018;15:20170949. https://doi.org/10.1098/rsif.2017.0949.
Chen XY, Butt AM, Mohd Amin MCI. Enhanced paracellular delivery of vaccine by hydrogel microparticles-mediated reversible tight junction opening for effective oral immunization. J Contr Release. 2019;311–312:50–64. https://doi.org/10.1016/j.jconrel.2019.08.031.
Bhatnagar P, Law JX, Ng SF. Chitosan reinforced with kenaf nanocrystalline cellulose as an effective carrier for the delivery of platelet lysate in the acceleration of wound healing. Polymers. 2021;4392(13):4392. https://doi.org/10.3390/POLYM13244392.
Jacob B, Park I-S, Bang J-K, Shin SY. Short KR-12 analogs designed from human cathelicidin LL-37 possessing both antimicrobial and antiendotoxic activities without mammalian cell toxicity. J Peptide Sci. 2013;19:700–7. https://doi.org/10.1002/psc.2552.
Liang C-C, Park AY, Guan J-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–33. https://doi.org/10.1038/nprot.2007.30.
Machado HA, Abercrombie JJ, You T, DeLuca PP, Leung KP. Release of a wound-healing agent from PLGA microspheres in a thermosensitive gel. Biomed Res Int. 2013;2013:387863. https://doi.org/10.1155/2013/387863.
Ali NH, Amin MCIM, Ng S-F. Sodium carboxymethyl cellulose hydrogels containing reduced graphene oxide (rGO) as a functional antibiofilm wound dressing. J Biomater Sci Polym Ed. 2019;30:629–45. https://doi.org/10.1080/09205063.2019.1595892.
Chereddy KK, Lopes A, Koussoroplis S, Payen V, Moia C, Zhu H, Sonveaux P, Carmeliet P, Rieux A, Vandermeulen G, Préat V. Combined effects of PLGA and vascular endothelial growth factor promote the healing of non-diabetic and diabetic wounds. Nanomedicine. 2015;11:1975–84. https://doi.org/10.1016/j.nano.2015.07.006.
McClements DJ. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv Colloid Interface Sci. 2018;253:1–22. https://doi.org/10.1016/j.cis.2018.02.002.
Feczkó T, Tóth J, Dósa GY, Gyenis J. Optimization of protein encapsulation in PLGA nanoparticles. Chem Eng Process Process Intens. 2011;50:757–65. https://doi.org/10.1016/j.cep.2011.06.008.
Lozeau LD, Grosha J, Smith IM, Stewart EJ, Camesano TA, Rolle MW. Alginate affects bioactivity of chimeric collagen binding LL37 antimicrobial peptides adsorbed to collagen-alginate wound dressings. ACS Biomater Sci Eng. 2020;6(6):3398–410. https://doi.org/10.1021/acsbiomaterials.0c00227.
Toppazzini M, Coslovi A, Boschelle M, Marsich E, Benincasa M, Gennaro R, et al. Can the interaction between the antimicrobial peptide LL-37 and alginate be exploited for the formulation of new biomaterials with antimicrobial properties? Carbohydr Polym. 2011;83(2):578–85. https://doi.org/10.1016/j.carbpol.2010.08.020.
Zarzycki R, Modrzejewska Z, Nawrotek K. Drug release from hydrogel matrices. Ecol Chem Eng S. 2010;17(2):117–36.
Nicolle L, Journot CMA, Gerber-Lemaire S. Chitosan functionalization: Covalent and non-covalent interactions and their characterization. Polymers (Basel). 2021;13(23):4118. https://doi.org/10.3390/polym13234118.
Dong L, Henderson A, Field C. Antimicrobial activity of single-walled carbon nanotubes suspended in different surfactants. J Nanotechnol. 2012;2012:928924. https://doi.org/10.1155/2012/928924.
Szymańska E, Winnicka K. Stability of chitosan—a challenge for pharmaceutical and biomedical applications. Mar Drugs. 2015;13:1819–46. https://doi.org/10.3390/md13041819.
Quesada HB, de Araújo TP, Vareschini DT, de Barros MASD, Gomes RG, Bergamasco R. Chitosan, alginate and other macromolecules as activated carbon immobilizing agents: A review on composite adsorbents for the removal of water contaminants. Int J Biol Macromol. 2020;164:2535–49. https://doi.org/10.1016/j.ijbiomac.2020.08.118.
Sidik AM, Othaman R, Anuar FH. The effect of molecular weight on the surface and permeation of poly(L-lactic acid)-poly(ethylene glycol) membrane with activated carbon filler. Sains Malays. 2018;47:1181–7.
Xu K, An N, Zhang H, Zhang Q, Zhang K, Hu X, Wu Y, Wu F, Xiao J, Zhang H, Peng R, Li H, Jia C. Sustained-release of PDGF from PLGA microsphere embedded thermo-sensitive hydrogel promoting wound healing by inhibiting autophagy. J Drug Deliv Sci Technol. 2020;55:101405. https://doi.org/10.1016/j.jddst.2019.101405.
Bernal-Chávez SA, Alcalá-Alcalá S, Cerecedo D, Ganem-Rondero A. Platelet lysate-loaded PLGA nanoparticles in a thermo-responsive hydrogel intended for the treatment of wounds. Eur J Pharm Sci. 2020;146:105231. https://doi.org/10.1016/j.ejps.2020.105231.
Yoo J, Won Y-Y. Phenomenology of the initial burst release of drugs from PLGA microparticles. ACS Biomater Sci Eng. 2020;6:6053–62. https://doi.org/10.1021/acsbiomaterials.0c01228.
Kaur T, Thirugnanam A. Effect of porous activated charcoal reinforcement on mechanical and in-vitro biological properties of polyvinyl alcohol composite scaffolds. J Mater Sci Technol. 2017;33:734–43. https://doi.org/10.1016/j.jmst.2016.06.020.
Nagant C, Pitts B, Nazmi K, Vandenbranden M, Bolscher JG, Stewart PS, Dehaye J-P. Identification of peptides derived from the human antimicrobial peptide LL-37 active against biofilms formed by pseudomonas aeruginosa using a library of truncated fragments. Antimicrob Agents Chemother. 2012;56:5698–708. https://doi.org/10.1128/aac.00918-12.
Morgera F, Vaccari L, Antcheva N, Scaini D, Pacor S, Tossi A. Primate cathelicidin orthologues display different structures and membrane interactions. Biochemical Journal. 2009;417:727–35. https://doi.org/10.1042/BJ20081726.
El Maguana Y, Elhadiri N, Bouchdoug M, Benchanaa M, Jaouad A. Activated carbon from prickly pear seed cake: Optimization of preparation conditions using experimental design and its application in dye removal. Int J Chem Eng. 2019;2019:8621951. https://doi.org/10.1155/2019/8621951.
Rosenfeld Y, Papo N, Shai Y. Endotoxin (Lipopolysaccharide) neutralization by innate immunity host-defense peptides: Peptide properties and plausible modes of action. J Biol Chem. 2006;281:1636–43. https://doi.org/10.1074/jbc.M504327200.
Cirioni O, Giacometti A, Ghiselli R, Bergnach C, Orlando F, Silvestri C, Mocchegiani F, Licci A, Skerlavaj B, Rocchi M, Saba V, Zanetti M, Scalise G. LL-37 protects rats against lethal sepsis caused by gram-negative bacteria. Antimicrob Agents Chemother. 2006; 50(5):1672–9. https://doi.org/10.1128/aac.50.5.1672-1679.2006.
Illsley MJ, Akhmetova A, Bowyer C, Nurgozhin T, Mikhalovsky SV, Farrer J, Dubruel P, Allan IU. Activated carbon-plasticised agarose composite films for the adsorption of thiol as a model of wound malodour. J Mater Sci Mater Med. 2017;28:154. https://doi.org/10.1007/s10856-017-5964-x.
Müller G, Winkler Y, Kramer A. Antibacterial activity and endotoxin-binding capacity of actisorb® silver 220. J Hospital Infect. 2003;53:211–4. https://doi.org/10.1053/jhin.2002.1369.
Tomasinsig L, Pizzirani C, Skerlavaj B, Pellegatti P, Gulinelli S, Tossi A, Di Virgilio F, Zanetti M. The human cathelicidin LL-37 modulates the activities of the P2X7 receptor in a structure-dependent manner. J Biol Chem. 2008;283:30471–81. https://doi.org/10.1074/jbc.M802185200.
Oudhoff MJ, Blaauboer ME, Nazmi K, Scheres N, Bolscher JGM, Veerman ECI. The role of salivary histatin and the human cathelicidin LL-37 in wound healing and innate immunity. Biol Chem. 2010;391:541–8. https://doi.org/10.1515/bc.2010.057.
Howling GI, Dettmar PW, Goddard PA, Hampson FC, Dornish M, Wood EJ. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials. 2001;22:2959–66. https://doi.org/10.1016/S0142-9612(01)00042-4.
Barańska-Rybak W, Sonesson A, Nowicki R, Schmidtchen A. Glycosaminoglycans inhibit the antibacterial activity of LL-37 in biological fluids. J Antimicrob Chemother. 2006;57:260–5. https://doi.org/10.1093/jac/dki460.
Ridyard KE, Overhage J. The potential of human peptide LL-37 as an antimicrobial and anti-biofilm agent. Antibiotics. 2021;10:650. https://doi.org/10.3390/antibiotics10060650.
Ke C-L, Deng F-S, Chuang C-Y, Lin C-H. Antimicrobial actions and applications of chitosan. Polymers (Basel). 2021;13:905. https://doi.org/10.3390/polym13060904.
Voljč T, Semenič D. Contribution of topical agents to wound healing. In: S. Aghaei (Ed.), Recent Advances in Wound Healing, IntechOpen, Rijeka, 2021: p Ch. 10. https://doi.org/10.5772/intechopen.97170.
Luo Y, McLean DTF, Linden GJ, McAuley DF, McMullan R, Lundy FT. The naturally occurring host defense peptide, LL-37, and its truncated mimetics KE-18 and KR-12 have selected biocidal and antibiofilm activities against candida albicans, staphylococcus aureus, and escherichia coli in vitro. Front Microbiol. 2017;8:544. https://doi.org/10.3389/fmicb.2017.00544.
Dean S, Bishop B, Van Hoek M. Susceptibility of pseudomonas aeruginosa biofilm to alpha-helical peptides: D-enantiomer of LL-37. Front Microbiol. 2011;2:128. https://doi.org/10.3389/fmicb.2011.00128.
Kang J, Dietz MJ, Li B. Antimicrobial peptide LL-37 is bactericidal against Staphylococcus aureus biofilms. PLoS ONE. 2019;14:e0216676. https://doi.org/10.1371/JOURNAL.PONE.0216676.
Overhage J, Campisano A, Bains M, Torfs EC, Rehm BH, Hancock RE. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Immun. 2008;76(9):4176–82. https://doi.org/10.1128/iai.00318-08.
Kai-Larsen Y, Lüthje P, Chromek M, Peters V, Wang X, Holm Å, Kádas L, Hedlund KO, Johansson J, Chapman MR, Jacobson SH, Römling U, Agerberth B, Brauner A. Uropathogenic escherichia coli modulates immune responses and its curli fimbriae interact with the antimicrobial peptide LL-37. PLoS Pathog. 2010;6:e1001010. https://doi.org/10.1371/JOURNAL.PPAT.1001010.
Begun J, Gaiani JM, Rohde H, Mack D, Calderwood SB, Ausubel FM, Sifri CD. Staphylococcal biofilm exopolysaccharide protects against caenorhabditis elegans immune defenses. PLoS Pathog. 2007;3:e57. https://doi.org/10.1371/journal.ppat.0030057.
Acknowledgements
Authors would like to thank the Faculty of Pharmacy, UKM for the research facilities support and special thanks to UKM Medical Molecular Biology Institute (UMBI) for supporting the C. elegans work.
Funding
This research is financially supported by the research grant no. GUP-2019–003, Universiti Kebangsaan Malaysia (UKM).
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology, S.-F. Ng., writing—original draft preparation, B.-Y. Lim., and S.-F. Ng.; review and editing, F.Azmi. and S.-F.Ng. funding acquisition, S.-F. Ng. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lim, BY., Azmi, F. & Ng, SF. LL37 Microspheres Loaded on Activated Carbon-chitosan Hydrogel: Anti-bacterial and Anti-toxin Wound Dressing for Chronic Wound Infections. AAPS PharmSciTech 25, 110 (2024). https://doi.org/10.1208/s12249-024-02826-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02826-6