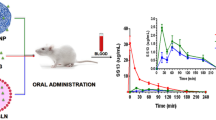
Abstract
Transport of oral nanocarriers across the GI epithelium necessitates transport across hydrophilic mucus layer and the hydrophobic epithelium. Based on hydrophobic-hydrophilic balance, Curcumin-Lipomer (lipid-polymer hybrid nanoparticles) comprising hydrophobic stearic acid and hydrophilic Gantrez™ AN 119 (Gantrez) were developed, by a radical in-situ approach, to successfully traverse both barriers. A monophasic preconcentrate (Cur-Pre) comprising Cur (Curcumin), stearic acid, Gantrez and stabilizers, prepared by simple solution, was added to an aqueous phase to instantaneously generate Curcumin-Lipomer (Cur-Lipo) of nanosize and high entrapment efficiency (EE). Cur-Lipo size and EE was optimized by Box-Behnken Design. Cur-Lipomers of varying hydrophobic-hydrophilic property obtained by varying the stearic acid: Gantrez ratio exhibited size in the range 200–400 nm, EE > 95% and spherical morphology as seen in the TEM. A decrease in contact angle and in mucus interaction, evident with increase in Gantrez concentration, indicated an inverse corelation with hydrophilicity, while a linear corelation was observed for mucopenetration and hydrophilicity. Cur-SLN (solid lipid nanoparticles) which served as the hydrophobic reference revealed contact angle > 90°, maximum interaction with mucus and minimal mucopenetration. The ex-vivo permeation study through chicken ileum, revealed maximum permeation with Cur-Lipo1 and comparable and significantly lower permeation of Cur-Lipo1-D and Cur-SLN proposing the importance of balancing the hydrophobic-hydrophilic property of the nanoparticles. A 1.78-fold enhancement in flux of hydrophobic Cur-SLN, with no significant change in permeation of the hydrophilic Cur-Lipomers (p > 0.05) following stripping off the mucosal layer was observed. This reiterated the significance of hydrophobic-hydrophilic balance as a promising strategy to design nanoformulations with superior permeation across the GI barrier.
Graphical Abstract







Similar content being viewed by others
References
Ejazi SA, Louisthelmy R, Maisel K. Mechanisms of nanoparticle transport across intestinal tissue: an oral delivery perspective. ACS Nano. 2023;17(14):13044–61. https://doi.org/10.1021/acsnano.3c02403.
Mittal R, Patel AP, Jhaveri VM, Kay S-IS, Debs LH, Parrish JM, et al. Recent advancements in nanoparticle based drug delivery for gastrointestinal disorders. Expert Opin Drug Deliv. 2018;15(3):301–18. https://doi.org/10.1080/17425247.2018.1420055.
Bachhav SS, Dighe VD, Kotak D, Devarajan PV. Rifampicin Lipid-Polymer hybrid nanoparticles (LIPOMER) for enhanced Peyer’s patch uptake. Int J Pharm. 2017;532(1):612–22. https://doi.org/10.1016/j.ijpharm.2017.09.040.
Roger E, Lagarce F, Garcion E, Benoit J-P. Biopharmaceutical parameters to consider in order to alter the fate of nanocarriers after oral delivery. Nanomedicine. 2010;5(2):287–306. https://doi.org/10.2217/nnm.09.110.
Shahbazi M-A, Santos HA. Improving oral absorption via drug-loaded nanocarriers: absorption mechanisms, intestinal models and rational fabrication. Curr Drug Metab. 2013;14(1):28–56. https://doi.org/10.2174/138920013804545133.
Mo R, Jiang T, Di J, Tai W, Gu Z. Emerging micro-and nanotechnology based synthetic approaches for insulin delivery. Chem Soc Rev. 2014;43(10):3595–629. https://doi.org/10.1039/C3CS60436E.
Shan W, Zhu X, Tao W, Cui Y, Liu M, Wu L, et al. Enhanced oral delivery of protein drugs using zwitterion-functionalized nanoparticles to overcome both the diffusion and absorption barriers. ACS Appl Mater Interfaces. 2016;8(38):25444–53. https://doi.org/10.1021/acsami.6b08183.
Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64(6):557–70. https://doi.org/10.1016/j.addr.2011.12.009.
Huckaby JT, Lai SK. PEGylation for enhancing nanoparticle diffusion in mucus. Adv Drug Deliv Rev. 2018;124:125–39. https://doi.org/10.1016/j.addr.2017.08.010.
Yu M, Xu L, Tian F, Su Q, Zheng N, Yang Y, et al. Rapid transport of deformation-tuned nanoparticles across biological hydrogels and cellular barriers. Nat Commun. 2018;9(1):2607. https://doi.org/10.1038/s41467-018-05061-3.
Menzel C, Bernkop-Schnürch A. Enzyme decorated drug carriers: targeted swords to cleave and overcome the mucus barrier. Adv Drug Deliv Rev. 2018;124:164–74. https://doi.org/10.1016/j.addr.2017.10.004.
Leal J, Smyth HD, Ghosh D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int J Pharm. 2017;532(1):555–72. https://doi.org/10.1016/j.ijpharm.2017.09.018.
Kirch J. The role of fluid dynamics, microstructure and mucociliary clearance in the micro-and macroscopic barrier properties of pulmonary mucus. 2012. https://doi.org/10.22028/D291-22833.
Hagesaether E, Adamczak MI, Hiorth M, Tho I. Characterization of Bioadhesion, Mucin‐interactions and Mucosal Permeability of Pharmaceutical Nano‐and Microsystems. Charact Pharm Nano Microsyst. 2021:171–205. https://doi.org/10.1002/9781119414018.ch5.
Anselmo AC, Gokarn Y, Mitragotri S. Non-invasive delivery strategies for biologics. Nat Rev Drug Discovery. 2019;18(1):19–40. https://doi.org/10.1038/nrd.2018.183.
Macedo AS, Castro PM, Roque L, Thomé NG, Reis CP, Pintado ME, et al. Novel and revisited approaches in nanoparticle systems for buccal drug delivery. J Control Release. 2020;320:125–41. https://doi.org/10.1016/j.jconrel.2020.01.006.
Liu C, Jiang X, Gan Y, Yu M. Engineering nanoparticles to overcome the mucus barrier for drug delivery: Design, evaluation and state-of-the-art. Medicine in Drug Discovery. 2021;12:100110. https://doi.org/10.1016/j.medidd.2021.100110.
Yang M, Lai SK, Wang Y-Y, Zhong W, Happe C, Zhang M, et al. Biodegradable nanoparticles composed entirely of safe materials that rapidly penetrate human mucus. Angewandte Chemie (International ed in English). 2011;50(11):2597. https://doi.org/10.1002/anie.201006849.
Ensign LM, Schneider C, Suk JS, Cone R, Hanes J. Mucus penetrating nanoparticles: biophysical tool and method of drug and gene delivery. Adv Mater. 2012;24(28):3887–94. https://doi.org/10.1002/adma.201201800.
Gao Y, He Y, Zhang H, Zhang Y, Gao T, Wang J-H, et al. Zwitterion-functionalized mesoporous silica nanoparticles for enhancing oral delivery of protein drugs by overcoming multiple gastrointestinal barriers. J Colloid Interface Sci. 2021;582:364–75. https://doi.org/10.1016/j.jcis.2020.08.010.
Khutoryanskiy VV. Beyond PEGylation: alternative surface-modification of nanoparticles with mucus-inert biomaterials. Adv Drug Deliv Rev. 2018;124:140–9. https://doi.org/10.1016/j.addr.2017.07.015.
Schneider CS, Xu Q, Boylan NJ, Chisholm J, Tang BC, Schuster BS, et al. Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci Adv. 2017;3(4):e1601556. https://doi.org/10.1126/sciadv.1601556.
Wu L, Shan W, Zhang Z, Huang Y. Engineering nanomaterials to overcome the mucosal barrier by modulating surface properties. Adv Drug Deliv Rev. 2018;124:150–63. https://doi.org/10.1016/j.addr.2017.10.001.
Zhang X, Cheng H, Dong W, Zhang M, Liu Q, Wang X, et al. Design and intestinal mucus penetration mechanism of core-shell nanocomplex. J Control Release. 2018;272:29–38. https://doi.org/10.1016/j.jconrel.2017.12.034.
Fang L, Wang L, Yao Y, Zhang J, Wu X, Li X, et al. Micro-and nano-carrier systems: The non-invasive and painless local administration strategies for disease therapy in mucosal tissues. Nanomedicine. 2017;13(1):153–71. https://doi.org/10.1016/j.nano.2016.08.025.
Forier K, Messiaen A-S, Raemdonck K, Deschout H, Rejman J, De Baets F, et al. Transport of nanoparticles in cystic fibrosis sputum and bacterial biofilms by single-particle tracking microscopy. Nanomedicine. 2013;8(6):935–49. https://doi.org/10.2217/nnm.12.129.
Valamla B, Thakor P, Phuse R, Dalvi M, Kharat P, Kumar A, et al. Engineering drug delivery systems to overcome the vaginal mucosal barrier: Current understanding and research agenda of mucoadhesive formulations of vaginal delivery. J Drug Deliv Sci Technol. 2022;70:103162. https://doi.org/10.1016/j.jddst.2022.103162.
Hu S, Yang Z, Wang S, Wang L, He Q, Tang H, et al. Zwitterionic polydopamine modified nanoparticles as an efficient nanoplatform to overcome both the mucus and epithelial barriers. Chem Eng J. 2022;428:132107. https://doi.org/10.1016/j.cej.2021.132107.
Jahagirdar PS, Gupta PK, Kulkarni SP, Devarajan PV. Polymeric curcumin nanoparticles by a facile in situ method for macrophage targeted delivery. Bioeng Transl Med. 2019;4(1):141–51. https://doi.org/10.1002/btm2.10112.
Joshi HA, Patwardhan RS, Sharma D, Sandur SK, Devarajan PV. Pre-clinical evaluation of an innovative oral nano-formulation of baicalein for modulation of radiation responses. Int J Pharm. 2021;595:120181. https://doi.org/10.1016/j.ijpharm.2020.120181.
Poinard B, Kamaluddin S, Tan AQQ, Neoh KG, Kah JCY. Polydopamine coating enhances mucopenetration and cell uptake of nanoparticles. ACS Appl Mater Interfaces. 2019;11(5):4777–89. https://doi.org/10.1021/acsami.8b18107.
Grießinger J, Dünnhaupt S, Cattoz B, Griffiths P, Oh S, Igómez SB, et al. Methods to determine the interactions of micro-and nanoparticles with mucus. Eur J Pharm Biopharm. 2015;96:464–76. https://doi.org/10.1016/j.ejpb.2015.01.005.
Hu S, Pei X, Duan L, Zhu Z, Liu Y, Chen J, et al. A mussel-inspired film for adhesion to wet buccal tissue and efficient buccal drug delivery. Nat Commun. 2021;12(1):1689. https://doi.org/10.1038/s41467-021-21989-5.
Westerhout J, Bellmann S, van Ee R, Havenaar R, Leeman W, Steeg E, et al. Prediction of oral absorption of nanoparticles from biorelevant matrices using a combination of physiologically relevant in vitro and ex vivo models. J Food Chem Nanotechnol. 2017;3(4):111–9. https://doi.org/10.17756/jfcn.2017-046.
Behrens I, Pena AIV, Alonso MJ, Kissel T. Comparative uptake studies of bioadhesive and non-bioadhesive nanoparticles in human intestinal cell lines and rats: the effect of mucus on particle adsorption and transport. Pharm Res. 2002;19:1185–93. https://doi.org/10.1023/A:1019854327540.
Jindal AB, Devarajan PV. Asymmetric lipid–polymer particles (LIPOMER) by modified nanoprecipitation: role of non-solvent composition. Int J Pharm. 2015;489(1–2):246–51. https://doi.org/10.1016/j.ijpharm.2015.04.073.
Kapse SV, Gaikwad RV, Samad A, Devarajan PV. Self nanoprecipitating preconcentrate of tamoxifen citrate for enhanced bioavailability. Int J Pharm. 2012;429(1–2):104–12. https://doi.org/10.1016/j.ijpharm.2012.02.042.
John R, Dalal B, Shankarkumar A, Devarajan PV. Innovative betulin nanosuspension exhibits enhanced anticancer activity in a triple negative breast cancer cell line and Zebrafish angiogenesis model. Int J Pharm. 2021;600: 120511. https://doi.org/10.1016/j.ijpharm.2021.120511.
Todke PA, Devarajan PV. In-silico approach as a tool for selection of excipients for safer amphotericin B nanoformulations. J Control Release. 2022;349:756–64. https://doi.org/10.1016/j.jconrel.2022.07.030.
D’Souza AA, Devarajan PV. Bioenhanced oral curcumin nanoparticles: role of carbohydrates. Carbohyd Polym. 2016;136:1251–8. https://doi.org/10.1016/j.carbpol.2015.10.021.
Ferreira SC, Bruns R, Ferreira HS, Matos GD, David J, Brandão G, et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal Chim Acta. 2007;597(2):179–86. https://doi.org/10.1016/j.aca.2007.07.011.
Homayouni A, Sadeghi F, Varshosaz J, Garekani HA, Nokhodchi A. Promising dissolution enhancement effect of soluplus on crystallized celecoxib obtained through antisolvent precipitation and high pressure homogenization techniques. Colloids Surf B. 2014;122:591–600. https://doi.org/10.1016/j.colsurfb.2014.07.037.
Crick CR, Parkin IP. Preparation and characterisation of super-hydrophobic surfaces. Chem Eur J. 2010;16(12):3568–88. https://doi.org/10.1002/chem.200903335.
Azioune A, Chehimi MM, Miksa B, Basinska T, Slomkowski S. Hydrophobic protein− polypyrrole interactions: The role of van der Waals and Lewis acid− base forces as determined by contact angle measurements. Langmuir. 2002;18(4):1150–6. https://doi.org/10.1021/la010444o.
Chater PI, Wilcox MD, Pearson JP. Efficacy and safety concerns over the use of mucus modulating agents for drug delivery using nanoscale systems. Adv Drug Deliv Rev. 2018;124:184–92. https://doi.org/10.1016/j.addr.2017.12.006.
Nordgård CT, Draget KI. Co association of mucus modulating agents and nanoparticles for mucosal drug delivery. Adv Drug Deliv Rev. 2018;124:175–83. https://doi.org/10.1016/j.addr.2018.01.001.
Svensson O, Thuresson K, Arnebrant T. Interactions between drug delivery particles and mucin in solution and at interfaces. Langmuir. 2008;24(6):2573–9. https://doi.org/10.1021/la702680x.
Takeuchi H, Thongborisute J, Matsui Y, Sugihara H, Yamamoto H, Kawashima Y. Novel mucoadhesion tests for polymers and polymer-coated particles to design optimal mucoadhesive drug delivery systems. Adv Drug Deliv Rev. 2005;57(11):1583–94. https://doi.org/10.1016/j.addr.2005.07.008.
de Sousa IP, Steiner C, Schmutzler M, Wilcox MD, Veldhuis GJ, Pearson JP, et al. Mucus permeating carriers: formulation and characterization of highly densely charged nanoparticles. Eur J Pharm Biopharm. 2015;97:273–9. https://doi.org/10.1016/j.ejpb.2014.12.024.
Griffiths PC, Cattoz B, Ibrahim MS, Anuonye JC. Probing the interaction of nanoparticles with mucin for drug delivery applications using dynamic light scattering. Eur J Pharm Biopharm. 2015;97:218–22. https://doi.org/10.1016/j.ejpb.2015.05.004.
Ren Y, Wu W, Zhang X. The feasibility of oral targeted drug delivery: Gut immune to particulates? Acta Pharmaceutica Sinica B. 2023;13(6):2544–58. https://doi.org/10.1016/j.apsb.2022.10.020.
Dos Santos AM, Carvalho SG, Meneguin AB, Sábio RM, Gremião MPD, Chorilli M. Oral delivery of micro/nanoparticulate systems based on natural polysaccharides for intestinal diseases therapy: Challenges, advances and future perspectives. J Control Release. 2021;334:353–66. https://doi.org/10.1016/j.jconrel.2021.04.026.
Froehlich E, Roblegg E. Mucus as barrier for drug delivery by nanoparticles. J Nanosci Nanotechnol. 2014;14(1):126–36. https://doi.org/10.1166/jnn.2014.9015.
Teubl BJ, Meindl C, Eitzlmayr A, Zimmer A, Fröhlich E, Roblegg E. In-vitro permeability of neutral polystyrene particles via buccal mucosa. Small. 2013;9(3):457–66. https://doi.org/10.1002/smll.201201789.
Funding
The financial support for Junior Research Fellowship from Department of Atomic Energy (DAE-ICT), Government of India to Esha S. Attar is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Esha Attar: Investigation, Methodology, Validation, Writing—original draft, S. Jayakumar- Writing—review & editing, Padma V. Devarajan: Conceptualization, Supervision, Resources, funding acquisition and writing and editing the final draft.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Attar, E.S., Jayakumar, S. & Devarajan, P.V. Oral In-Situ Nanoplatform with Balanced Hydrophobic-Hydrophilic Property for Transport Across Gastrointestinal Mucosa. AAPS PharmSciTech 25, 113 (2024). https://doi.org/10.1208/s12249-024-02824-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02824-8