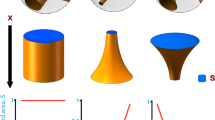
Abstract
Hydrocortisone (HC) is the optimal drug for adolescents diagnosed with congenital adrenal hyperplasia (CAH). Because traditional dosage regimens HC are inconvenient, our study used fused deposition modeling (FDM) three-dimensional (3D) printing technology to solve the problems caused by traditional preparations. First, we designed a core–shell structure tablet with an inner instant release component and an outer delayed release shell. The instant release component was Kollicoat IR: glycerol (GLY): HC = 76.5:13.5:10. Then, we used Affinisol® HPMC 15LV to realize delayed release. Furthermore, we investigated the relationship between the thickness of the delayed release shell and the delayed release time, and an equation was derived through binomial regression analysis. Based on that equation, a novel triple pulsatile tablet with an innovative structure was devised. The tablet was divided into three components, and the drug was released multiple times at different times. The dose and release rate of the tablets can be adjusted by modifying the infill rate of the printing model. The results indicated that the triple pulsatile tablet exhibited desirable release behavior in vitro. Moreover, the physicochemical properties of the drug, excipients, filaments, and tablets were characterized. All these results indicate that the FDM 3D printing method is a convenient technique for producing preparations with intricate structures.
Graphical Abstract






Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Nermoen I, Husebye ES, Myhre AG, Lovas K. Classic congenital adrenal hyperplasia. Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin, ny raekke. 2017;137(7):540-3. https://doi.org/10.4045/tidsskr.16.0376.
Gong C, Cheng X, Yan L, Li Z, Gou P, Tang F, et al. Clinical analysis of 28 cases of neonatal congenital adrenal hyperplasia Sichuan Medical Journal. 2021;42(03):240–4. https://doi.org/10.16252/j.cnki.issn1004-0501-2021.03.006..
Idkowiak J, Elhassan YS, Mannion P, Smith K, Webster R, Saraff V, et al. Causes, patterns and severity of androgen excess in 487 consecutively recruited pre- and post-pubertal children. Eur J Endocrinol. 2019;180(3):213–21. https://doi.org/10.1530/eje-18-0854.
Lu WL, Ma XY, Zhang J, Wang JQ, Zhang TT, Ye L, et al. Clinical and molecular characterization of 10 Chinese children with congenital adrenal hyperplasia due to 11beta-hydroxylase deficiency. World J Pediatr. 2023. https://doi.org/10.1007/s12519-023-00739-1.
Pofi R, Ji XC, Krone NP, Tomlinson JW. Long-term health consequences of congenital adrenal hyperplasia. Clin Endocrinol. 2023. https://doi.org/10.1111/cen.14967.
Debor B, Bechtold-Dalla Pozza S, Reisch N, Schmidt H, Dubinski I. Effect of complete suppression of androstenedione on auxological development in prepubertal patients with classical congenital adrenal hyperplasia. J Pediatr Endocrinol Metab: JPEM. 2023. https://doi.org/10.1515/jpem-2023-0169.
Finkielstain GP, Rey RA. Challenges in managing disorders of sex development associated with adrenal dysfunction. Expert Rev Endocrinol Metab. 2023;18(5):427–39. https://doi.org/10.1080/17446651.2023.2256393.
Bridwell RE, April MD. Adrenal emergencies. Emerg Med Clin North Am. 2023;41(4):795–808. https://doi.org/10.1016/j.emc.2023.06.006.
Frigerio S, Carosi G, Ferrante E, Sala E, Polledri E, Fustinoni S, et al. Effects of the therapy shift from cortisone acetate to modified-release hydrocortisone in a group of patients with adrenal insufficiency. Front Endocrinol. 2023;14. https://doi.org/10.3389/fendo.2023.1093838.
Puglisi S, Perini AME, Botto C, Oliva F, Terzolo M. Long-term consequences of Cushing’s syndrome: a systematic literature review. J Clin Endocrinol Metab. 2023. https://doi.org/10.1210/clinem/dgad453.
Papadakis GE, de Kalbermatten B, Dormoy A, Salenave S, Trabado S, Vieira-Pinto O, et al. Impact of Cushing’s syndrome on the gonadotrope axis and testicular functions in men. Hum Reprod. 2023. https://doi.org/10.1093/humrep/dead187.
Whitaker MJ, Debono M, Ross RJ. Developing oral chronotherapy for cortisol replacement in congenital adrenal hyperplasia. Clin Endocrinol. 2023. https://doi.org/10.1111/cen.14976.
Derendorf H, Mollmann H, Barth J, Mollmann C, Tunn S, Krieg M. Pharmacokinetics and oral bioavailability of hydrocortisone. J Clin Pharmacol. 1991;31(5):473–6. https://doi.org/10.1002/j.1552-4604.1991.tb01906.x.
Bonner JJ, Burt H, Johnson TN, Whitaker MJ, Porter J, Ross RJ. Development and verification of an endogenous PBPK model to inform hydrocortisone replacement dosing in children and adults with cortisol deficiency. Eur J Pharm Sci. 2021;165. https://doi.org/10.1016/j.ejps.2021.105913..
Hens B, Corsetti M, Bermejo M, Löbenberg R, González PM, Mitra A, et al. "Development of fixed dose combination products" workshop report: considerations of gastrointestinal physiology and overall development strategy. AAPS J. 2019;21(4). https://doi.org/10.1208/s12248-019-0346-6.
Sunil SA, Srikanth MV, Rao NS, Murthy KVR. Chronotherapeutic drug delivery from indomethacin compression coated tablets for early morning pain associated rheumatoid arthritis. Curr Drug Deliv. 2013;10(1):109–21.
Bhat BB, Mehta CH, Suresh A, Velagacherla V, Nayak UY. Controlled release technologies for chronotherapy: current status and future perspectives. Curr Pharm Des. 2023;29(14):1069–91. https://doi.org/10.2174/1381612829666230423144232.
Charoenthai N, Wickramanayaka A, Sungthongjeen S, Puttipipatkhachorn S. Use of cassava starch nanocrystals to make a robust rupturable pulsatile release pellet. J Drug Deliv Sci Technol. 2018;47:283–90. https://doi.org/10.1016/j.jddst.2018.07.026.
Penhasi A, Gomberg M. Design and development of an innovative water insoluble film-coating combination for oral pulsatile drug delivery. J Drug Deliv Sci Technol. 2018;43:274–82. https://doi.org/10.1016/j.jddst.2017.10.019.
Adam D. Emerging science of chronotherapy offers big opportunities to optimize drug delivery. Proc Natl Acad Sci USA. 2019;116(44):21957–9. https://doi.org/10.1073/pnas.1916118116.
Field EA, Brookes V, Tyldesley WR. Recurrent aphthous ulceration in children–a review. Int J Pediatr Dent. 1992;2(1):1–10.
Sarvan MS, Nori LP. Personalized medicine: a new normal for therapeutic success. Indian J Pharm Sci. 2021;83(3):416–29. https://doi.org/10.36468/pharmaceutical-sciences.790.
Amekyeh H, Tarlochan F, Billa N. Practicality of 3D printed personalized medicines in therapeutics. Front Pharmacol. 2021;12. https://doi.org/10.3389/fphar.2021.646836.
Algahtani MS. Assessment of pharmacist’s knowledge and perception toward 3D printing technology as a dispensing method for personalized medicine and the readiness for implementation. Pharmacy (Basel, Switzerland). 2021;9(1). https://doi.org/10.3390/pharmacy9010068.
Beer N, Hegger I, Kaae S, De Bruin ML, Genina N, Alves TL, et al. Scenarios for 3D printing of personalized medicines - a case study. Exploratory Res Clin Soc Pharm. 2021;4:100073. https://doi.org/10.1016/j.rcsop.2021.100073.
Englezos K, Wang LX, Tan ECK, Kang LF. 3D printing for personalised medicines: implications for policy and practice. Int J Pharm. 2023;635. https://doi.org/10.1016/j.ijpharm.2023.122785..
Cui MS, Pan H, Su YP, Fang DY, Qiao S, Ding PT, et al. Opportunities and challenges of three-dimensional printing technology in pharmaceutical formulation development. Acta Pharmaceutica Sinica B. 2021;11(8):2488–504. https://doi.org/10.1016/j.apsb.2021.03.015.
Kotta S, Nair A, Alsabeelah N. 3D printing technology in drug delivery: recent progress and application. Curr Pharm Des. 2018;24(42):5039–48. https://doi.org/10.2174/1381612825666181206123828.
Wang JW, Zhang Y, Aghda NH, Pillai AR, Thakkar R, Nokhodchi A, et al. Emerging 3D printing technologies for drug delivery devices: current status and future perspective. Adv Drug Deliv Rev. 2021;174:294–316. https://doi.org/10.1016/j.addr.2021.04.019.
Long J, Gholizadeh H, Lu J, Bunt C, Seyfoddin A. Application of fused deposition modelling (FDM) method of 3D printing in drug delivery. Curr Pharm Des. 2017;23(3):433–9. https://doi.org/10.2174/1381612822666161026162707.
Muhindo D, Elkanayati R, Srinivasan P, Repka MA, Ashour EA. Recent advances in the applications of additive manufacturing (3D printing) in drug delivery: a comprehensive review (vol 24, 57, 2023). AAPS PharmSciTech. 2023;24(3):75. https://doi.org/10.1208/s12249-023-02542-7.
Gioumouxouzis CI, Karavasili C, Fatouros DG. Recent advances in pharmaceutical dosage forms and devices using additive manufacturing technologies. Drug Disc Today. 2019;24(2):636–43. https://doi.org/10.1016/j.drudis.2018.11.019.
Alhijjaj M, Belton P, Qi S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur J Pharm Biopharm. 2016;108:111–25. https://doi.org/10.1016/j.ejpb.2016.08.016.
Chen H, Li X, Gong Y, Bu T, Wang X, Pan H. Unidirectional drug release from 3D printed personalized buccal patches using FDM technology. International Journal of Pharmaceutics. 2023:123382. https://doi.org/10.1016/j.ijpharm.2023.123382.
Goyanes A, Kobayashi M, Martínez-Pacheco R, Gaisford S, Basit AW. Fused-filament 3D printing of drug products: microstructure analysis and drug release characteristics of PVA-based caplets. Int J Pharm. 2016;514(1):290–5. https://doi.org/10.1016/j.ijpharm.2016.06.021.
Sharma V, Shaik KM, Choudhury A, Kumar P, Kala P, Sultana Y, et al. Investigations of process parameters during dissolution studies of drug loaded 3D printed tablets. Proc Inst Mech Eng Part H-J Eng Med. 2021;235(5):523–9. https://doi.org/10.1177/0954411921993582.
Mahmood F, Hussain A, Arshad MS, Abbas N, Irfan M, Qamar N, et al. Effect of solublising aids on the entrapment of loratidine in pre-fabricated PVA filaments used for FDM based 3D-printing. Acta Pol Pharm. 2020;77(1):175–82. https://doi.org/10.32383/appdr/113596.
Melocchi A, Uboldi M, Cerea M, Foppoli A, Maroni A, Moutaharrik S, et al. A graphical review on the escalation of fused deposition modeling (FDM) 3D printing in the pharmaceutical field. J Pharm Sci. 2020;109(10):2943–57. https://doi.org/10.1016/j.xphs.2020.07.011.
Chai X, Chai H, Wang X, Yang J, Li J, Zhao Y, et al. Fused deposition modeling (FDM) 3D printed tablets for intragastric floating delivery of domperidone. Sci Rep. 2017;7:2829. https://doi.org/10.1038/s41598-017-03097-x.
Jamroz W, Kurek M, Szafraniec-Szczesny J, Czech A, Gawlak K, Knapik-Kowalczuk J, et al. Speed it up, slow it down...an issue of bicalutamide release from 3D printed tablets. Eur J Pharm Sci. 2020;143:105169. https://doi.org/10.1016/j.ejps.2019.105169.
Zhang PL, Xu PC, Chung S, Bandari S, Repka MA. Fabrication of bilayer tablets using hot melt extrusion-based dual-nozzle fused deposition modeling 3D printing. Int J Pharm. 2022;624. https://doi.org/10.1016/j.ijpharm.2022.121972.
Gioumouxouzis CI, Tzimtzimis E, Katsamenis OL, Dourou A, Markopoulou C, Bouropoulos N, et al. Fabrication of an osmotic 3D printed solid dosage form for controlled release of active pharmaceutical ingredients. Eur J Pharm Sci. 2020;143:105176. https://doi.org/10.1016/j.ejps.2019.105176.
Annaji M, Mita N, Heard J, Kang X, Poudel I, Fasina O, et al. 3D-printed capsaicin-loaded injectable implants for targeted delivery in obese patients. AAPS PharmSciTech. 2023;24(7):200. https://doi.org/10.1208/s12249-023-02647-z.
Cui YD, Chen B, Wang G, Wang JT, Liu B, Zhu L, et al. Partial talar replacement with a novel 3D printed prosthesis. Comput Assist Surg. 2023;28(1). https://doi.org/10.1080/24699322.2023.2198106..
Gupta T, Ghosh SB, Bandyopadhyay-Ghosh S, Sain M. Is it possible to 3D bioprint load-bearing bone implants? A critical review. Biofabrication. 2023;15(4). https://doi.org/10.1088/1758-5090/acf6e1..
Gioumouxouzis CI, Baklavaridis A, Katsamenis OL, Markopoulou CK, Bouropoulos N, Tzetzis D, et al. A 3D printed bilayer oral solid dosage form combining metformin for prolonged and glimepiride for immediate drug delivery. Eur J Pharm Sci. 2018;120:40–52. https://doi.org/10.1016/j.ejps.2018.04.020.
Melocchi A, Uboldi M, Briatico-Vangosa F, Moutaharrik S, Cerea M, Foppoli A, et al. The Chronotopic (TM) system for pulsatile and colonic delivery of active molecules in the era of precision medicine: feasibility by 3D printing via fused deposition modeling (FDM). Pharmaceutics. 2021;13(5). https://doi.org/10.3390/pharmaceutics13050759.
Tidau M, Finke JH. Modified release kinetics in dual filament 3D printed individualized oral dosage forms. Eur J Pharm Sci. 2022;175. https://doi.org/10.1016/j.ejps.2022.106221..
Eleftheriadis GK, Ritzoulis C, Bouropoulos N, Tzetzis D, Andreadis DA, Boetker J, et al. Unidirectional drug release from 3D printed mucoadhesive buccal films using FDM technology: in vitro and ex vivo evaluation. Eur J Pharm Biopharm. 2019;144:180–92. https://doi.org/10.1016/j.ejpb.2019.09.018.
Chen H, Li X, Gong Y, Bu TS, Wang XY, Pan H. Unidirectional drug release from 3D printed personalized buccal patches using FDM technology. Int J Pharm. 2023;645. https://doi.org/10.1016/j.ijpharm.2023.123382..
McDonagh T, Belton P, Qi S. Manipulating drug release from 3D printed dual-drug loaded polypills using challenging polymer compositions. Int J Pharm. 2023;637. https://doi.org/10.1016/j.ijpharm.2023.122895..
Okwuosa TC, Pereira BC, Arafat B, Cieszynska M, Isreb A, Alhnan MA. Fabricating a shell-core delayed release tablet using dual FDM 3D printing for patient-centred therapy. Pharm Res. 2017;34(2):427–37. https://doi.org/10.1007/s11095-016-2073-3.
Yang TL, Stogiannari M, Janeczko S, Khoshan M, Lin YY, Isreb A, et al. Towards point-of-care manufacturing and analysis of immediate-release 3D printed hydrocortisone tablets for the treatment of congenital adrenal hyperplasia. Int J Pharm. 2023;642. https://doi.org/10.1016/j.ijpharm.2023.123072..
Li R, Pan Y, Chen D, Xu XY, Yan GR, Fan TY. Design, preparation and in vitro evaluation of core-shell fused deposition modelling 3D-printed verapamil hydrochloride pulsatile tablets. Pharmaceutics. 2022;14(2). https://doi.org/10.3390/pharmaceutics14020437..
Parulski C, Bya LA, Goebel J, Servais AC, Lechanteur A, Evrard B. Development of 3D printed mini-waffle shapes containing hydrocortisone for children’s personalized medicine. Int J Pharm. 2023;642. https://doi.org/10.1016/j.ijpharm.2023.123131..
Ayyoubi S, van Kampen EEM, Kocabas LI, Parulski C, Lechanteur A, Evrard B, et al. 3D printed, personalized sustained release cortisol for patients with adrenal insufficiency. Int J Pharm. 2023;630. https://doi.org/10.1016/j.ijpharm.2022.122466..
Gupta SS, Solanki N, Serajuddin ATM. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion, IV: Affinisol™ HPMC HME Polymers. AAPS PharmSciTech. 2016;17(1):148–57. https://doi.org/10.1208/s12249-015-0426-6.
Melocchi A, Parietti F, Maroni A, Foppoli A, Gazzaniga A, Zema L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int J Pharm. 2016;509(1–2):255–63. https://doi.org/10.1016/j.ijpharm.2016.05.036.
Wu H, Liu YH, Ci TY, Ke X. Application of HPMC HME polymer as hot melt extrusion carrier in carbamazepine solid dispersion. Drug Dev Ind Pharm. 2020;46(12):1911–8. https://doi.org/10.1080/03639045.2020.1821045.
Svoboda R, Nevyhostena M, Machackova J, Vaculik J, Knotkova K, Chromcikova M, et al. Thermal degradation of Affinisol HPMC: optimum processing temperatures for hot melt extrusion and 3D printing. Pharm Res. 2023;40(9):2253–68. https://doi.org/10.1007/s11095-023-03592-z.
Fouad EA, El-Badry M, Neau SH, Alanazi FK, Alsarra IA. Technology evaluation: Kollicoat IR. Expert Opin Drug Deliv. 2011;8(5):693–703. https://doi.org/10.1517/17425247.2011.566266.
Xu L, Li SM, Wang Y, Wei M, Yao HM, Sunada H. Improvement of dissolution rate of ibuprofen by solid dispersion systems with Kollicoat IR using a pulse combustion dryer system. J Drug Deliv Sci Technol. 2009;19(2):113–8. https://doi.org/10.1016/s1773-2247(09)50018-4.
Kolter K, Dashevsky A, Irfan M, Bodmeier R. Polyvinyl acetate-based film coatings. Int J Pharm. 2013;457(2):470–9. https://doi.org/10.1016/j.ijpharm.2013.08.077.
Mandati P, Dumpa N, Alzahrani A, Nyavanandi D, Narala S, Wang HH, et al. Hot-melt extrusion-based fused deposition modeling 3D printing of atorvastatin calcium tablets: impact of shape and infill density on printability and performance. Aaps Pharmscitech. 2022;24(1). https://doi.org/10.1208/s12249-022-02470-y.
Patel NG, Serajuddin ATM. Improving drug release rate, drug-polymer miscibility, printability and processability of FDM 3D-printed tablets by weak acid-base interaction. Int J Pharm. 2023;632. https://doi.org/10.1016/j.ijpharm.2022.122542.
Wu JT, Chen N, Wang Q. Preparation of novel thermoplastic poly(vinyl alcohol) with improved processability for fused deposition modeling. Polym Adv Technol. 2018;29(5):1447–55. https://doi.org/10.1002/pat.4256.
Saikia J, Devi TG, Karlo T. A combined spectroscopic and quantum chemical approach to study the molecular interaction between anti-inflammatory drug Hydrocortisone and amino acid L-Phenylalanine. J Mol Struct. 2023;1286. https://doi.org/10.1016/j.molstruc.2023.135546..
Author information
Authors and Affiliations
Contributions
Hao Chen: conceptualization, methodology, investigation, writing—original draft; Kai Zheng: software, validation, data curation; Tianshi Bu: resources, project administration, formal analysis, writing—review and editing; Xin LI: writing—review and editing, visualization; Xiangyu Wang: writing—review and editing; Hao Pan: conceptualization, methodology, supervision, funding acquisition.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, H., Zheng, K., Bu, T. et al. Fabrication of 3D-Printed Hydrocortisone Triple Pulsatile Tablet Using Fused Deposition Modelling Technology. AAPS PharmSciTech 25, 58 (2024). https://doi.org/10.1208/s12249-024-02757-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02757-2