Abstract
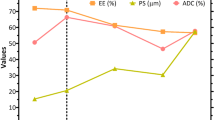
The present work explores a data mining approach to study the fabrication of prednisolone-loaded chitosan nanoparticles and their properties. Eight PLC formulations were prepared using an automated adaptation of the antisolvent precipitation method. The PLCs were characterized using dynamic light scattering, infrared spectroscopy, and drug release studies. Results showed that that the effective diameter, loading capacity, encapsulation efficiency, zeta potential, and polydispersity of the PLCs were influenced by the concentration and molecular weight of chitosan. The drug release studies showed that PLCs exhibited significant dissolution enhancement compared to pure prednisolone crystals. Principal components analysis and partial least squares regression were applied to the infrared spectra and the DLS data to extract higher-order interactions and correlations between the critical quality attributes and the diameter of the PLCs. Principal components revealed that the spectra clustered according to the type of material, with PLCs forming a separate cluster from the raw materials and the physical mix. PLS was successful in predicting the ED of the PLCs from the FTIR spectra with R2 = 0.98 and RMSE = 27.18. The present work demonstrates that data mining techniques can be useful tools for obtaining deeper insights into the fabrication and properties of PLCs, and for optimizing their quality and performance. It also suggests that FTIR spectroscopy can be a rapid and non-destructive method for predicting the ED of PLCs.









Similar content being viewed by others
Abbreviations
- PLC:
-
Prednisolone-loaded chitosan nanoparticles
- PRED:
-
Prednisolone
- HMW:
-
High molecular weight
- MMW:
-
Medium molecular weight
- ED:
-
Effective diameter
- LC:
-
Loading capacity
- EE:
-
Encapsulation efficiency
- ZP:
-
Zeta potential
- PD:
-
Polydispersity
- ATR–FTIR:
-
Attenuated total reflectance–Fourier transform infrared spectroscopy1
- DLS:
-
Dynamic light scattering
- PCA:
-
Principal component analysis
- PLS:
-
Partial least squares regression
- R2:
-
Coefficient of determination
- RMSE:
-
Residual mean square error
- PAT:
-
Process analytical technologies
- NIR:
-
Near-infrared
- MW:
-
Molecular weight
- MWCO:
-
Molecular weight cutoff
- LDA:
-
Linear discriminate analysis
- LOO:
-
Leave one out
- AI:
-
Artificial intelligence
References
Bentwich I. Pharma’s bio-AI revolution. Drug Discov Today. 2023;28: 103515.
Jiang J, Ma X, Ouyang D, Williams RO. Emerging artificial intelligence (AI) technologies used in the development of solid dosage forms. Pharmaceutics. 2022;14:2257.
Singh I, Juneja P, Kaur B, Kumar P. Pharmaceutical applications of chemometric techniques. ISRN Anal Chem. 2013;2013:1–13.
Lee LC, Liong CY, Jemain AA. A Contemporary review on data preprocessing (DP) practice strategy in ATR-FTIR spectrum. Chemom Intell Lab Syst. 2017;163:64–75.
Feelders A, Daniels H, Holsheimer M. Methodological and practical aspects of data mining in the product development process. Inf Manag. 2000;37:271–81.
Yao H, Vancoillie J, D’Hondt M, Wynendaele E, Bracke N, De SB. An analytical quality by design (Aqbd) approach for a L-asparaginase activity method. J Pharm Biomed Anal. 2016;117:232–9.
Rantanen J, Khinast J. The future of pharmaceutical manufacturing sciences. J Pharm Sci. 2015;104:3612–38.
Nasereddin J, Shakib M. Ira: a free and open-source Fourier transform infrared (FTIR) data analysis widget for pharmaceutical applications. Anal Lett. 2023;56:2637–48.
Biancolillo A, Marini F. Chemometric methods for spectroscopy-based pharmaceutical analysis. Front Chem. 2018;6:1–14.
Syafri S, Jaswir I, Yusof F, Rohman A, Hamidi D. The use Of GC-MS and FTIR spectroscopy coupled with multivariate analysis for the detection of red ginger oil adulteration. Rasayan J Chem. 2022;15:2231–6.
Beć KB, Grabska J, Huck CW. In silico NIR spectroscopy – A review. Molecular fingerprint, interpretation of calibration models, understanding of matrix effects and instrumental difference. Spectrochim Acta - Part A Mol Biomol Spectrosc. 2022;279:1–18.
Nandiyanto ABD, Oktiani R, Ragadhita R. How to read and interpret FTIR spectroscope of organic material. Indones J Sci Technol. 2019;4:97–118.
Huang T, Wang S, He K. Quality control for fused deposition modeling based additive manufacturing: Current research and future trends. Proc 2015 1st Int Conf Reliab Syst Eng ICRSE. 2015;2015.
Charoo NA, Ali AA. Quality risk management in pharmaceutical development. Drug Dev Ind Pharm. 2013;39:947–60.
Kousiatza C, Karalekas D. In-situ monitoring of strain and temperature distributions during fused deposition modeling process. Mater Des. 2016;97:400–6.
Steffens FE. What is principal components analysis? Nat Biotechnol. 1983;26:303–4.
Alhijjaj M, Nasereddin J, Belton P, Qi S. Impact of processing parameters on the quality of pharmaceutical solid dosage forms produced by fused deposition modeling (FDM). Pharmaceutics. 2019;11:1–21.
Lestari D, Rohman A, Syofyan S, Yuliana ND, Abu Bakar NKB, Hamidi D. Analysis of beef meatballs with rat meat adulteration using Fourier transform infrared (FTIR) spectroscopy in combination with chemometrics. Int J Food Prop. 2022;25:1446–57.
Baker MJ, Gazi E, Brown MD, Shanks JH, Gardner P, Clarke NW. FTIR-based spectroscopic analysis in the identification of clinically aggressive prostate cancer. Br J Cancer. 2008;99:1859–66.
Nasereddin JM, Wellner N, Alhijjaj M, Belton P, Qi S. Development of a simple mechanical screening method for predicting the feedability of a pharmaceutical FDM 3D printing filament. Pharm Res. 2018;35:150.
Sharifi A. Partial least squares-regression (PLS-regression). In: Chemometrics. 11th Natl Conf Achiev Chem Chem Eng. 2018. pp. 305–8.
Stacey P, Clegg F, Sammon C. Multicomponent measurement of respirable quartz, kaolinite and coal dust using Fourier transform infrared spectroscopy (FTIR): a comparison between partial least squares and principal component regressions. Ann Work Expo Heal. 2022;66:644–55.
Singh G, Kumar D, Sharma D, Singh M, Kaur S. Q-Absorbance ratio spectrophotometric method for the simultaneous estimation of prednisolone and 5-amino salicylic acid in tablet dosage form. J Appl Pharm Sci. 2012;2:222–6.
Iftikhar SY, Iqbal FM, Hassan W, Nasir B, Sarwar AR. Desirability combined response surface methodology approach for optimization of prednisolone acetate loaded chitosan nanoparticles and in-vitro assessment. Mater Res Express. 2020;7:115004.
Luque-Alcaraz AG, Lizardi-Mendoza J, Goycoolea FM, Higuera-Ciapara I, Argüelles-Monal W. Preparation of chitosan nanoparticles by nanoprecipitation and their ability as a drug nanocarrier. RSC Adv. 2016;6:59250–6.
Toplak M, Birarda G, Read S, Sandt C, Rosendahl SM, Vaccari L, et al. Infrared Orange: connecting hyperspectral data with machine learning. Synchrotron Radiat News. 2017;30:40–5.
Basu B, Aviya KR, Bhattacharya A. Development and characterization of mouth dissolving tablets of prednisolone. J Pharm Investig. 2014;44:79–102.
Madni A, Kashif PM, Nazir I, Tahir N, Rehman M, Khan MI, et al. Drug-polymer interaction studies of cytarabine loaded chitosan nanoparticles. J Chem Soc Pakistan. 2017;39:1045–54.
Lin J, Huang J, Chen J, Lin Z, Ou Y, Yao L, et al. Low cytotoxic D-mannitol isolated from the industrial wastewater of Agaricus bisporus. J Food Nutr Res. 2016;4:610–4.
Zaid Alkilani A, Hamed R, Abdo H, Swellmeen L, Basheer HA, Wahdan W, et al. Formulation and evaluation of azithromycin-loaded niosomal gel: optimization, in vitro studies, rheological characterization, and cytotoxicity study. ACS omega. 2022;7:39782–93.
Queiroz MF, Melo KRT, Sabry DA, Sassaki GL, Rocha HAO. Does the use of chitosan contribute to oxalate kidney stone formation? Mar Drugs. 2014;13:141–58.
O’Sullivan B, Glennon B. Application of in situ FBRM and ATR-FTIR to the monitoring of the polymorphic transformation of D-mannitol. Org Process Res Dev. 2005;9:884–9.
Sharma VK, Kalonia DS. Effect of vacuum drying on protein-mannitol interactions: the physical state of mannitol and protein structure in the dried state. AAPS PharmSciTech. 2004;5:58–69.
Oktay AN, Ilbasmis-Tamer S, Han S, Uludag O, Celebi N. Preparation and in vitro / in vivo evaluation of flurbiprofen nanosuspension-based gel for dermal application. Eur J Pharm Sci. 2020;155: 105548.
Chen YD, Liang ZY, Cen YY, Zhang H, Han MG, Tian YQ, et al. Development Of oral dispersible tablets containing prednisolone nanoparticles for the management of pediatric asthma. Drug Des Devel Ther. 2015;9:5815–25.
Albuquerque G, Eisemann M, Lehmann D, Theisel H, Magnor M. Improving the visual analysis of high-dimensional datasets using quality measures. In: IEEE Conference on Visual Analytics Science and Technology (VAST). 2010. pp. 19–26. https://doi.org/10.1109/VAST.2010.5652433
Angelini M, Blasilli G, Lenti S, Palleschi A, Santucci G. Towards enhancing RadViz analysis and interpretation. In: 2019 IEEE Vis Conf VIS 2019. Institute of Electrical and Electronics Engineers Inc.; 2019. p. 226–30.
Rubio-Sánchez M, Raya L, Díaz F, Sanchez A. A comparative study between Radviz and star coordinates. IEEE Trans Vis Comput Graph. 2016;22:619–28.
Zaid Alkilani A, Nimrawi S, Al-Nemrawi NK, Nasereddin J. Microneedle-assisted transdermal delivery of amlodipine besylate loaded nanoparticles. Drug Dev Ind Pharm. 2022;48:322–32.
Suttiponparnit K, Jiang J, Sahu M, Suvachittanont S, Charinpanitkul T, Biswas P. Role Of surface area, primary particle size, and crystal phase on titanium dioxide nanoparticle dispersion properties. Nanoscale Res Lett. 2011;6:1–8.
Hamdallah SI, Zoqlam R, Erfle P, Blyth M, Alkilany AM, Dietzel A, et al. Microfluidics for pharmaceutical nanoparticle fabrication: The truth and the myth. Int J Pharm. 2020;584:119408.
Godugu C, Doddapaneni R, Patel AR, Singh R, Mercer R, Singh M. Novel gefitinib formulation with improved oral bioavailability in treatment of A431 skin carcinoma. Pharm Res. 2016;33:137–54.
Palanisamy M, Khanam J. Solid dispersion of prednisolone: solid state characterization and improvement of dissolution profile. Drug Dev Ind Pharm. 2011;37:373–86.
Marsac PJ, Shamblin SL, Taylor LS. Theoretical and practical approaches for prediction of drug-polymer miscibility and solubility. Pharm Res. 2006;23:2417–26.
El-Gindy A, Hadad GM. Chemometrics in pharmaceutical analysis: an introduction, review, and future perspectives. J AOAC Int. 2012;95:609–23.
Wold S, Sjöström M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemom Intell Lab Syst. 2001;58:109–30.
Baker MJ, Trevisan J, Bassan P, Bhargava R, Butler HJ, Dorling KM, et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat Protoc. 2014;9:1771–91.
Zhang B, Nasereddin J, McDonagh T, von Zeppelin D, Gleadall A, Alqahtani F, et al. Effects of porosity on drug release kinetics of swellable and erodible porous pharmaceutical solid dosage forms fabricated by hot melt droplet deposition 3D printing. Int J Pharm. 2021;604:120626.
Leyk E, Wesolowski M. Interactions between paracetamol and hypromellose in the solid state. Front Pharmacol. 2019;10:14.
Garg U, Chauhan S, Nagaich U, Jain N. Current advances in chitosan nanoparticles based drug delivery and targeting. Tabriz Univ Med Sci. 2019;7:113–7.
Jisha KDMS. Chitosan nanoparticles preparation and applications. Environ Chem Lett. 2018;16:101–12.
Morgen M, Bloom C, Beyerinck R, Bello A, Song W, Wilkinson K, et al. Polymeric nanoparticles for increased oral bioavailability and rapid absorption using celecoxib as a model of a low-solubility, high-permeability drug. Pharm Res. 2012;29:427–40.
Alkilani AZ, Nasereddin J, Hamed R, Nimrawi S, Hussein G, Abo-Zour H, et al. Beneath the skin: a review of current trends and future prospects of transdermal drug delivery systems. Pharmaceutics. 2022;14:1–35.
Jermain SV, Brough C, Williams RO. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery – an update. Int J Pharm. 2018;535:379–92.
Gigliobianco MR, Casadidio C, Censi R, Di Martino P. Nanocrystals of poorly soluble drugs: drug bioavailability and physicochemical stability. Pharmaceutics. 2018;10:134.
Herdiana Y, Wathoni N, Shamsuddin S, Muchtaridi M. Drug release study of the chitosan-based nanoparticles. Heliyon. 2022;8:E08674.
Liang J, Yan H, Puligundla P, Gao X, Zhou Y, Wan X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: a review. Food Hydrocoll. 2017;69:286–92.
Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, et al. Pubchem In 2021: new data content and improved web interfaces. Nucleic Acids Res. 2021;49:D1388–95.
Funding
This project was graciously funded by the Zarqa University Deanship of Scientific Research (#15, Damascus Highway, Zarqa, 13110, Jordan).
Author information
Authors and Affiliations
Contributions
JN: primary supervision, experimental procedures, writing of the manuscript. RAW: experimental procedure. AZK: supervision, manuscript review. AAK: NANOPARTICLE Characterization, manuscript review. MN: nanoparticle characterization, manuscript review. WAD: analytical method validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nasereddin, J., Al Wadi, R., Zaid Al-Kilani, A. et al. The Use of Data Mining for Obtaining Deeper Insights into the Fabrication of Prednisolone-Loaded Chitosan Nanoparticles. AAPS PharmSciTech 25, 38 (2024). https://doi.org/10.1208/s12249-024-02756-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02756-3