Abstract
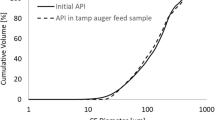
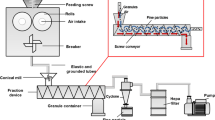
Previous work demonstrated that roller compaction of a 40%w/w theophylline–loaded formulation resulted in granulate consisting of un-compacted fractions which were shown to constitute between 34 and 48%v/v of the granulate dependent on processing conditions. The active pharmaceutical ingredient (API) primary particle size within the un-compacted fraction was also shown to have undergone notable size reduction. The aim of the current work was to test the hypothesis that the observations may be more indicative of the relative compactability of the API due to the formulation being above the percolation threshold. This was done by assessing the impact of varied API loads in the formulation on the non-granulated fraction of the final granulate and the extent of attrition of API particles within the non-granulated fraction. The influence of processing conditions for all formulations was also investigated. The results verify that the observations, both of this study and the previous work, are not a consequence of exceeding the percolation threshold. The volume of un-compacted material within the granulate samples was observed to range between 34.7 and 65.5% depending on the API load and roll pressure, whilst the API attrition was equivalent across all conditions.
Graphical Abstract




Similar content being viewed by others
References
Nesarikar VV, Patel C, Early W, Vatsaraj N, Sprockel O, Jerzweski R. Roller compaction process development and scale up using Johanson model calibrated with instrumented roll data. Int J Pharm. 2012;436(1–2):486–507.
Leane M, Pitt K, Reynolds G, Group MCSW. A proposal for a drug product Manufacturing Classification System (MCS) for oral solid dosage forms. Pharm Dev Technol. 2015;20(1):12–21.
Kleinebudde P. Roll compaction/dry granulation: pharmaceutical applications. Eur J Pharm Biopharm. 2004;58(2):317–26.
Gamble JF, Tobyn M, Dennis AB, Shah T. Roller compaction: application of an in-gap ribbon porosity calculation for the optimization of downstream granule flow and compactability characteristics. Pharm Dev Technol. 2010;15(3):223–9.
Wiedey R, Šibanc R, Wilms A, Kleinebudde P. How relevant is ribbon homogeneity in roll compaction/dry granulation and can it be influenced? Eur J Pharm Biopharm. 2018;133:232–9.
Sun CC, Kleinebudde P. Mini review: mechanisms to the loss of tabletability by dry granulation. Eur J Pharm Biopharm. 2016;106:9–14.
Perez-Gandarillas L, Perez-Gago A, Mazor A, Kleinebudde P, Lecoq O, Michrafy A. Effect of roll-compaction and milling conditions on granules and tablet properties. Eur J Pharm Biopharm. 2016;106:38–49.
Souihi N, Reynolds G, Tajarobi P, Wikström H, Haeffler G, Josefson M, et al. Roll compaction process modeling: transfer between equipment and impact of process parameters. Int J Pharm. 2015;484(1–2):192–206.
Herting MG, Kleinebudde P. Roll compaction/dry granulation: effect of raw material particle size on granule and tablet properties. Int J Pharm. 2007;338(1–2):110–8.
Inghelbrecht S, Remon JP. Reducing dust and improving granule and tablet quality in the roller compaction process. Int J Pharm. 1998;171(2):195–206.
Hwang K-M, Kim S-Y, Nguyen T-T, Cho C-H, Park E-S. Use of roller compaction and fines recycling process in the preparation of erlotinib hydrochloride tablets. Eur J Pharm Sci. 2019;131:99–110.
Chattoraj S, Daugherity P, McDermott T, Olsofsky A, Roth WJ, Tobyn M. Sticking and picking in pharmaceutical tablet compression: an IQ Consortium review. J Pharm Sci. 2018;107(9):2267–82.
Gamble JF, Akseli I, Ferreira AP, Leane M, Thomas S, Tobyn M, et al. Morphological distribution mapping: utilisation of modelling to integrate particle size and shape distributions. Int J Pharm. 2023;635: 122743.
Leane M, Pitt K, Reynolds G, Anwar J, Charlton S, Crean A, et al. A proposal for a drug product Manufacturing Classification System (MCS) for oral solid dosage forms. Pharm Dev Technol. 2015;20(1):12–21.
Scherließ R, Bock S, Bungert N, Neustock A, Valentin L. Particle engineering in dry powders for inhalation. Eur J Pharm Sci. 2022;172: 106158.
Chattoraj S, Sun CC. Crystal and particle engineering strategies for improving powder compression and flow properties to enable continuous tablet manufacturing by direct compression. J Pharm Sci. 2018;107(4):968–74.
Gamble J, Jones J, Tobyn M. Understanding the effect of API changes in pharmaceutical processing. Eur Pharm Rev. 2017;22(1):20–2.
MacLeod CS, Muller FL. On the fracture of pharmaceutical needle-shaped crystals during pressure filtration: case studies and mechanistic understanding. Org Process Res Dev. 2012;16(3):425–34.
Lekhal A, Girard K, Brown M, Kiang S, Khinast J, Glasser B. The effect of agitated drying on the morphology of l-threonine (needle-like) crystals. Int J Pharm. 2004;270(1–2):263–77.
Taylor L, Papadopoulos D, Dunn P, Bentham A, Dawson N, Mitchell J, et al. Predictive milling of pharmaceutical materials using nanoindentation of single crystals. Org Process Res Dev. 2004;8(4):674–9.
Hamilton P, Littlejohn D, Nordon A, Sefcik J, Slavin P, Andrews J, et al. Investigation of factors affecting isolation of needle-shaped particles in a vacuum-agitated filter drier through non-invasive measurements by Raman spectrometry. Chem Eng Sci. 2013;101:878–85.
Fukunaka T, Golman B, Shinohara K. Batch grinding kinetics of ethenzamide particles by fluidized-bed jet-milling. Int J Pharm. 2006;311(1):89–96.
Kulkarni SS, Janssen PHM, Dickhoff BHJ. The impact of material chemistry and morphology on attrition behavior of excipients during high shear blending. Powder Technol. 2023;427: 118694.
Mendez R, Velazquez C, Muzzio FJ. Effect of feed frame design and operating parameters on powder attrition, particle breakage, and powder properties. Powder Technol. 2012;229:253–60.
Paul S, Taylor LJ, Murphy B, Krzyzaniak JF, Dawson N, Mullarney MP, et al. Powder properties and compaction parameters that influence punch sticking propensity of pharmaceuticals. Int J Pharm. 2017;521(1–2):374–83.
Dembélé M, Hudon S, Simard J-S, Abatzoglou N, Gosselin R. A multivariate data analysis approach to tablet sticking on an industrial scale: a qualitative case study of an ibuprofen-based formulation. Pharm Dev Technol. 2022;27(10):1093–109.
Paul S, Taylor LJ, Murphy B, Krzyzaniak JF, Dawson N, Mullarney MP, et al. Toward a molecular understanding of the impact of crystal size and shape on punch sticking. Mol Pharm. 2020;17(4):1148–58.
Pishnamazi M, Casilagan S, Clancy C, Shirazian S, Iqbal J, Egan D, et al. Microcrystalline cellulose, lactose and lignin blends: process mapping of dry granulation via roll compaction. Powder Technol. 2019;341:38–50.
Sajjia M, Shirazian S, Egan D, Iqbal J, Albadarin AB, Southern M, et al. Mechanistic modelling of industrial-scale roller compactor ‘Freund TF-MINI model.’ Comput Chem Eng. 2017;104:141–50.
Reynolds G, Ingale R, Roberts R, Kothari S, Gururajan B. Practical application of roller compaction process modeling. Comput Chem Eng. 2010;34(7):1049–57.
Dec RT, Zavaliangos A, Cunningham JC. Comparison of various modeling methods for analysis of powder compaction in roller press. Powder Technol. 2003;130(1–3):265–71.
Hassan L, Jensen R, Megarry A, Blaabjerg LI. Simulation of roller compaction by combination of a compaction simulator and oscillating mill – a material sparing approach. Int J Pharm. 2023;644: 123281.
Kammrath BW, Koutrakos A, Castillo J, Langley C, Huck-Jones D. Morphologically-directed Raman spectroscopy for forensic soil analysis. Forensic Sci Int. 2018;285:e25–33.
Thomas BJ, Absar M, Delvadia R, Conti DS, Witzmann K, Guo C. Analytical method development for characterizing ingredient-specific particle size distributions of nasal spray suspension products. J Pharm Sci. 2021;110(7):2778–88.
Liu Q, Absar M, Saluja B, Guo C, Chowdhury B, Lionberger R, et al. Scientific considerations for the review and approval of first generic mometasone furoate nasal suspension spray in the United States from the bioequivalence perspective. AAPS J. 2019;21:1–6.
Gamble JF, Hoffmann M, Hughes H, Hutchins P, Tobyn M. Monitoring process induced attrition of drug substance particles within formulated blends. Int J Pharm. 2014;470(1–2):77–87.
Gamble JF, Dennis AB, Hutchins P, Jones JW, Musembi P, Tobyn M. Determination of process variables affecting drug particle attrition within multi-component blends during powder feed transmission. Pharm Dev Technol. 2017;22(7):904–9.
Clarke J, Gamble JF, Jones JW, Tobyn M, Dawson N, Davies C, et al. Determining the impact of roller compaction processing conditions on granule and API properties. AAPS PharmSciTech. 2020;21(6):218.
Leuenberger H. The application of percolation theory in powder technology. Adv Powder Technol. 1999;10(4):323–52.
Queiroz ALP, Faisal W, Devine K, Garvie-Cook H, Vucen S, Crean AM. The application of percolation threshold theory to predict compaction behaviour of pharmaceutical powder blends. Powder Technol. 2019;354:188–98.
Fuertes I, Miranda A, Millán M, Caraballo I. Estimation of the percolation thresholds in acyclovir hydrophilic matrix tablets. Eur J Pharm Biopharm. 2006;64(3):336–42.
Leuenberger H, Rohera B, Haas C. Percolation theory—a novel approach to solid dosage form design. Int J Pharm. 1987;38(1–3):109–15.
Leane M, Pitt K, Reynolds GK, Dawson N, Ziegler I, Szepes A, et al. Manufacturing Classification System in the real world: factors influencing manufacturing process choices for filed commercial oral solid dosage formulations, case studies from industry and considerations for continuous processing. Pharm Dev Technol. 2018;23(10):964–77.
Leuenberger H, Rohera BD, Haas C. Percolation theory — a novel approach to solid dosage form design. Int J Pharm. 1987;38(1):109–15.
Millán M, Caraballo I, Rabasco AM. The role of the drug/excipient particle size ratio in the percolation model for tablets. Pharm Res. 1998;15(2):216–20.
Allen T. Sampling of powders. In: Allen T, editor. Particle size measurement. Dordrecht: Springer, Netherlands; 1990. p. 1–40.
Gamble JF, Tobyn M, Hamey R. Application of image-based particle size and shape characterization systems in the development of small molecule pharmaceuticals. J Pharm Sci. 2015;104(5):1563–4.
Gamble JF, Chiu WS, Tobyn M. Investigation into the impact of sub-populations of agglomerates on the particle size distribution and flow properties of conventional microcrystalline cellulose grades. Pharm Dev Technol. 2011;16(5):542–8.
Kendall K. The impossibility of comminuting small particles by compression. Nature. 1978;272(5655):710–1.
Hoffmann M, Wray PS, Gamble JF, Tobyn M. Investigation into process-induced de-aggregation of cohesive micronised API particles. Int J Pharm. 2015;493(1–2):341–6.
Clarke J, Gamble JF, Jones JW, Tobyn M, Greenwood R, Ingram A. Alternative approach for defining the particle population requirements for static image analysis based particle characterization methods. Adv Powder Technol. 2019;30(5):920–9.
Acknowledgements
The authors would like to thank Tracy Gaebele, Srini Sridharan and Andrew Dennis (Bristol Myers Squibb).
Funding
The authors would like to acknowledge the EPSRC for funding.
Author information
Authors and Affiliations
Contributions
JC: conceptualization, analysis, interpretation, writing – review and editing. JFG: conceptualization, interpretation, supervision, writing – drafting, review and editing, final approval. JWJ: writing – review and editing. MT: conceptualization, writing – review and editing, final approval. AI: supervision, writing – drafting, review and editing, final approval. RG: supervision, writing – drafting, review and editing, final approval.
Corresponding author
Ethics declarations
Competing Interest
The authors declare no competing interests. The authors alone are responsible for the content and writing of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Clarke, J., Gamble, J.F., Jones, J.W. et al. Determining the Impact of Roller Compaction Processing Conditions on Granulate and API Properties: Impact of Formulation API Load. AAPS PharmSciTech 25, 24 (2024). https://doi.org/10.1208/s12249-024-02744-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02744-7