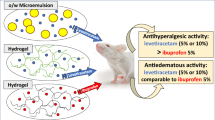
Abstract
Due to tenoxicam (TX)’s poor aqueous solubility (0.072 mg/ml), it is poorly absorbable in the GIT, and the long-term oral administration of TX may cause severe GIT disturbances. Topical administration of TX can help in bypassing the GIT adverse effects. Therefore, in the present work, we constructed different pluronic/lecithin organogels (PLOs) for topical delivery of TX. PLO was constructed simply via direct mixing of an aqueous pluronic solution with lecithin solution. The prepared PLO formulations were characterized for their physicochemical properties including pH, drug content, visual inspection, viscosity, and spreadability. Also, the in vitro release and kinetic studies were carried out to investigate the mechanism of drug release. Moreover, the in vivo studies were carried out by investigating the anti-inflammatory and analgesic activities using albino male rats. The results showed that the modified PLOs have good physicochemical properties. The viscosity of the modified gels is a direct proportionality with both lecithin and pluronic concentrations. Also, subsequently, the drug release rate is directly proportional to gel viscosity. Moreover, the in vivo studies showed that the modified PLOs (F19) showed a significant ( < 0.05%) paw edema inhibition and pain analgesia compared with other investigated groups. Also, the results indicated that the increase in dose is accompanied by higher activity and a longer duration of action which extended to 12 h. Hence, the modified PLOs are promising safe candidates or vehicles for effective TX loading with sustained delivery behavior.
Graphical abstract












Similar content being viewed by others
Data Availability
All data are contained within the article.
References
Nesseem DI, Eid SF, El-Houseny SS. Development of novel transdermal self-adhesive films for tenoxicam, an anti-inflammatory drug. Life Sciences. 2011;89:430–8. https://doi.org/10.1016/j.lfs.2011.06.026.
Gonzalez JP, Todd PA. Tenoxicam: a preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy. Drugs. 1987;34:289–310.
Vintiloiu A, Leroux J-C. Organogels and their use in drug delivery - a review. J Control Release. 2008;125:179–92.
Murdan S. Organogels in drug delivery. Expert Opin. 2005;2:29–39.
Garg T, Bilandi A, Kapoor B, Kumar S, Joshi R. Organogels: advanced and novel drug delivery system. Int Res J Pharm. 2011;2:15–21.
Wade A, Waller D. PJ Handbook of Pharmaceutical Excipients. 2nd ed. Washington DC: American Pharmaceutical Association; 1994.
Szuhaj, BF. Lecithins: Sources, manufacture, and uses. Champaign (Ill.): American Oil Chemists’ Society; 1989. VII, 283 p.: ill. AOCS monographs 12. https://lib.ugent.be/catalog/rug01:000220282.
Raut S, Bhadoriya SS, Uplanchiwar V, Mishra V, Gahane A, Jain SK. Lecithin organogel: a unique micellar system for the delivery of bioactive agents in the treatment of skin aging. Acta Pharm Sin B Elsevier. 2012;2:8–15. https://doi.org/10.1016/j.apsb.2011.12.005.
Smith J, Hong-Shum, Lily E. Food additives data book. 2nd ed. Food Res Int. 2011. https://doi.org/10.1002/9781444397741.ch8.
Kumar R, Katare OP. Lecithin organogels as a potential phospholipid-structured system for topical drug delivery: A review. AAPS PharmSciTech. 2005;6:298–310.
Motulsky A, Lafleur M, Couffin-Hoarau A-C, Hoarau D, Boury F, Benoit J-P, et al. Characterization and biocompatibility of organogels based on L-alanine for parenteral drug delivery implants. Biomaterials. 2005;26:6242–53.
Scartazzini R, Luisi PL. Organogels from lecithins. J Phys Chem. 1988;92:829–33.
Esposito E, Ravani L, Mariani P, Huang N, Boldrini P, Drechsler M, et al. Effect of nanostructured lipid vehicles on percutaneous absorption of curcumin. Eur J Pharm Biopharm. 2014;86:121–32. https://doi.org/10.1016/j.ejpb.2013.12.011. (Elsevier B.V.).
Mandal S, Snigdha SM, Krutika KS. Lecithin stabilized organogel: design and development for topical application of clobetasol propionate. Int J ChemTech Res. 2010;2:1214–9.
Shaikh IM, Jadhav SL, Jadhav KR, Kadam VJ, Pisal SS. Aceclofenac organogels: in vitro and in vivo characterization. Curr Drug Deliv. 2009;6:1–7.
Shaikh IM, Jadhav KR, Gide PS, Kadam VJ, Pisal SS. Topical delivery of aceclofenac from lecithin organogels: Preformulation study. Curr Drug Deliv. 2006;3:417–27.
García-Couce J, Tomás M, Fuentes G, Que I, Almirall A, Cruz LJ. Chitosan/Pluronic F127 thermosensitive hydrogel as an injectable dexamethasone delivery carrier. Gels. 2022;8:44. https://doi.org/10.3390/gels8010044.
Abdellatif AAH, Mohammed AM, Saleem I, Alsharidah M, Al Rugaie O, Ahmed F, et al. Smart injectable chitosan hydrogels loaded with 5-fluorouracil for the treatment of breast cancer. Pharmaceutics. 2022;14: 661. https://doi.org/10.3390/pharmaceutics14030661
Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130:98–106. https://doi.org/10.1016/j.jconrel.2008.04.013. (Elsevier B.V.).
Alsaab H, Bonam SP, Bahl D, Chowdhury P, Alexander K, Boddu SHS. Organogels in drug delivery: a special emphasis on pluronic lecithin organogels. J Pharm Pharm Sci. 2016;19:252–73.
Kabanov AV, Batrakova EV, Alakhov VY. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release. 2002;82:189–212.
Gu Z, Wang M, Fang Q, Zheng H, Wu F, Lin D, et al. Preparation and in vitro characterization of pluronic-attached polyamidoamine dendrimers for drug delivery. Drug Dev Ind Pharm. 2015;41:812–8.
Kabanov A, Zhu J, Alakhov V. Pluronic block copolymers for gene delivery. Adv Genet. 2005;53:231–61.
Crandall WT, inventor. Topical moisturizing composition and method. USA Patent 6 316 428. November 13, 2001.
Belgamwar VS, Pandey MS, Chauk DS, Surana SJ. Pluronic lecithin organogel. Asian J Pharm. 2008;2:134–8.
Sahoo S, Kumar N, Bhattacharya C, Sagiri SS, Jain K, Pal K, et al. Organogels: properties and applications in drug delivery. Des Monomers Polym. 2011;14:95–108.
Padilla M, Clark GT, Merrill RL. Topical medications for orofacial neuropathic pain: a review. J Am Dent Assoc. 2000;131:184–95. https://doi.org/10.14219/jada.archive.2000.0146. (American Dental Association).
Burnham R, Gregg R, Healy P, Steadward R. The effectiveness of topical diclofenac for lateral epicondylitis. Clin J Sport Med. 1998;8:78–81.
Richards H, Thomas CP, Bowen JL, Heard CM. In-vitro transcutaneous delivery of ketoprofen and polyunsaturated fatty acids from a pluronic lecithin organogel vehicle containing fish oil. J Pharm Pharmacol. 2006;58:903–8.
Stationwala R, Patidar A, Main P, Choukse AS, Agrawal S. Transdermal delivery of lornoxicam from pluronic lecithin organogel. Int J Chem Pharm Sci. 2011;2:32–7.
Mohammed AM, Faisal W, Saleh KI, Osman SK. Self-assembling organogels based on pluronic and lecithin for sustained release of etodolac: In vitro and in vivo correlation. Curr Drug Deliv. 2017;14:926–34.
Yassin TM, Saleh KI, Sarhan H, Osman SK. Formulation and evaluation of sorbitanmonostearate organogel as a topical delivery system for tenoxicam. Int J Innov Drug R&D. 2019;2:1–7.
Goindi S, Narula M, Kalra A. Microemulsion-based topical hydrogels of tenoxicam for treatment of arthritis. AAPS PharmSciTech. 2016;17:597–606. https://doi.org/10.1208/s12249-015-0383-0. (AAPS PharmSciTech).
Nandini D, Chauhan N, Chandra A, Pathak K. Effect of permeation enhancers on the release and permeation kinetics of oxytetracycline hydrochloride organogel formulations. J Young Pharm. 2009;1:285–9.
Osman SK, Yassin TM, Mohammed AM, Alfayomy AM, Abdellatif AA, Mahdi WA, et al. A novel approach for the availability and ocular delivery of tenoxicam potassium: Synthesis, characterization, and in vivo application. AAPS PharmSciTech. 2023;24:44. https://doi.org/10.1208/s12249-022-02487-3. (Springer International Publishing).
Costa P, Sousa Lobo JM. Evaluation of mathematical models describing drug release from estradiol transdermal systems. Drug Dev Ind Pharm. 2003;29:89–97.
Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–33.
Ritger PL, Peppas NA. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release. 1987;5:37–42.
Burnham KP, Anderson DR. Model selection and inference: a practical information-theoretic approach 2. J. Wildl. Manage. 2004.
Singhvi G, Singh M. Review: In vitro drug release characterization models. Int J Pharm Stud Res [Internet]. 2011;2:77–84. Available from: https://www.researchgate.net/publication/285447890_Review_In_vitro_Drug_Release_Characterization_Models
Kumar L, Verma R. In vitro evaluation of topical gel prepared using natural polymer. Int J Drug Deliv. 2010;2:58–63.
Lehman PA, Raney SG, Franz TJ. Percutaneous absorption in man: in vitro-in vivo correlation. Skin Pharmacol Physiol. 2011;24:224–30.
Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60:110–1.
Varelas CG, Dixon DG, Steiner CA. Zero-order release from biphasic polymer hydrogels. J Control Release. 1995;34:185–92.
Donbrow M, Samuelov Y. Zero order drug delivery from double-layered porous films: release rate profiles from ethyl cellulose, hydroxypropyl cellulose and polyethylene glycol mixtures. J Pharm Pharmacol. 1980;32:463–70.
Gibaldi M, Feldman S. Establishment of sink conditions in dissolution rate determinations—theoretical considerations and application to nondisintegrating dosage forms. J Pharm Sci. 1960;23:247–52.
Wagner JG. Interpretation of percent dissolved-time plots derived from in vitro testing of conventional tablets and capsules. J Pharm Sci. 1969;58:1253–7.
Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspension. J Pharm Sci. 1961;50:874–5.
Higuchi T. Mechanism of sustained-action medication-theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–9.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35.
Auda SH, El-Rasoul SA, Ahmed MM, et al. In-vitro release and in-vivo performance of tolmetin from different topical gel formulations. J Pharm Investig. 2015;45:311–7. https://doi.org/10.1007/s40005-015-0174-3.
Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Exp Biol Med. 1962;3:544–7.
Reddy DN, Udupa N. Formulation and evaluation of oral and transdermal preparations of flurbiprofen and piroxicam incorporated with different carriers. Drug Dev Ind Pharm. 1993;19:843–52.
Shahiwala A, Misra A. Studies in topical application of niosomally entrapped Nimesulide. J Pharm Pharm Sci. 2002;5:220–5.
Habib FS, Hassan MA, Abou El Ela AESF, El Sayeh F, Raheem R. Different topical formulations of ketorolac tromethamine for anti-inflammatory application and clinical efficacy. Dig J Nanomater Biostructures. 2014;9:705–19.
Winter CA, Risley EA, Nuss GW. Anti-inflammatory and antipyretic activities of indomethacin, 1-(p-chlorobenzoyl)-5- methoxy-2-methyl-indole-3-acetic acid. J pharmacol ExperTherapeutics. 1963;141:369–76.
Koster R. Acetic acid for analgesic screening. Fed proc. 1959;18:412.
Dambisya YM, Lee TL, Sathivulu V, Mat Jais AM. Influence of temperature, pH and naloxone on the antinociceptive activity of Channa striatus (haruan) extracts in mice. J Ethnopharmacol. 1999;66:181–6.
Vogel GH. Drug discovery and evaluation: pharmacological assays. 3rd ed. Germany: Springer Science & Business Media; 2002.
Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82:377–90.
Boddu SH, Bonam SP, Wei Y, Alexander K. Preparation and in vitro evaluation of a pluronic lecithin organogel containing ricinoleic acid for transdermal delivery. Int J Pharm Compd. 2014;18:256–61.
Shchipunov YA, Shumilina EV. Lecithin bridging by hydrogen bonds in the organogel. Mater Sci Eng C. 1995;3:43–50.
Chawla A, Sharma P, Pawar P. Eudragit S-100 coated sodium alginate microspheres of naproxen sodium: Formulation, optimization and in vitro evaluation. Acta Pharm. 2012;62:529–45.
Purohit B, Gupta N, Jain S. Formulation and evaluation of diclofenac sodium organogel. Res J Pharm Technol. 2013;6:375–8.
Aiyalu R, Govindarjan A, Ramasamy A. Formulation and evaluation of topical herbal gel for the treatment of arthritis in animal model. Brazilian J Pharm Sci. 2016;52:493–507.
Asghar A, Aamir MN, Sheikh FA, Ahmad N, Elsherif MA, Bukhari SNA. Co-combination of pregabalin and withania coagulans-extract-loaded topical gel aleviates allodynia and hyperalgesia in the chronic sciatic nerve constriction injury for neuropathic pain in animal model. Molecules. 2022;27:4433.
Plaza-Villegas F, Heir G, Markman S, Khan J, Noma N, Benoliel R, et al. Topical pregabalin and diclofenac for the treatment of neuropathic orofacial pain in rats. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:449–56. https://doi.org/10.1016/j.oooo.2012.05.002. (Elsevier).
Jhawat V, Gupta S, Saini V. Formulation and evaluation of novel controlled release of topical pluronic lecithin organogel of mefenamic acid. Drug Deliv. 2016;23:3573–81.
Abu-Elyazid SK, Kassem AA, Samy AM, Gomaa ME. Evaluation of skin permeation and pharmacological effects of tenoxicam nanoemulsión in topical formulations. Asian J Pharm Heal Sci. 2011;1:99–105.
Acknowledgements
The authors are thankful to the Researchers Supporting Project number (RSP2024R146) at King Saud University, Riyadh, Saudi Arabia. The authors would like to thank the Deanship of Scientific Research at Shaqra University for supporting this work.
Funding
This research was funded by Researchers Supporting Project number (RSP2024R146) at King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization, S.K.O. Data curation, T.M.Y., A.M.M., F.A., and S.K.O. Formal analysis, A.M.M., T.M.Y., F.A., and S.K.O. Investigation, A.A.A. and S.K.O. Methodology, S.K.O., T.M.Y., and A.M.M. Project administration, M.A.E.H., W.A.M., S.A., and S.K.O. Resources, W.A.M., S.A., and S.K.O. Software, A.M.M., W.A.M., and S.A. Validation, M.A.E.H. and F.A. Visualization, T.M.Y. and S.K.O. Writing—original draft, T.M.Y and S.K.O. Writing—review and editing, T.M.Y, F.A., A.A.A., A.A., W.A.M., S.A., and S.K.O.
Corresponding authors
Ethics declarations
Ethics Approval
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of Faculty of Pharmacy, Al-Azhar University, with approval number AZ-AS/PH/3/C/2021, for studies involving animals.
Informed Consent
Not applicable.
Conflicts of Interest
The authors declare no competing interests.
Additional information
Communicated by Nisarg Modi, Yousuf Mohammed, and Lakshmi Raghavan
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Osman, S.K., Yassin, T.M., Abdelzaher, A. et al. Self-assembling Organogels Loaded with Tenoxicam for Local Intensive Pain and Inflammation Cure: In Vitro and In Vivo Correlation. AAPS PharmSciTech 25, 18 (2024). https://doi.org/10.1208/s12249-024-02742-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02742-9