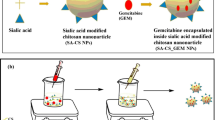
Abstract
Targeted gemcitabine (GEB) loaded 5-N-acetyl-neuraminic acid (Neu5Ac) assembled chitosan nanoparticles (CA-NPs) were formulated by ionotropic gelation process and evaluated for physicochemical and morphological characterization, in vitro and in vivo studies in A-549 cells and lung cancer mice model, respectively. The mean diameter of GEB-CA-Neu5Ac-NPs determined by dynamic light scattering was 161.16 ± 7.70 nm with a polydispersity index (PDI) value of 0.303 ± 0.011 and its zeta potential and entrapment efficiency (%EE) were 40.3 ± 3.45 mv and 66.11 ± 1.94%, respectively. The in vitro cellular uptake studies showed that glycan receptor-targeted nanoparticles deliver significantly more amount (p < 0.001) of GEB into the A-549 lung cancerous cells than non-targeted nanoparticles. The cytotoxicity study using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay clearly demonstrated that GEB-CA-Neu5Ac-NPs have lower IC50 value (6.39 ± 3.78 µg/ml) than others groups that showed that the greater lung cancerous cells inhibition potential of targeted nanoparticles. The in vivo biodistribution of the GEB-loaded 5-N-acetyl-neuraminic acid conjugated chitosan nanoparticles was revealed that targeted nanoparticles showed higher accumulation and retention for an extended period of time due to the active targeting ability of Neu5Ac to glycan receptors. Histopathological examination showed significant recovery in the physiological architecture upon administration of targeted nanoparticles. The glycan receptor-targeted nanoparticles treated groups showed a significant decline in the number of metastatic lung epithelial cells, as compared to the untreated positive control group (p < 0.001) confirming higher anticancer efficacy of the GEB-CA-Neu5Ac-NPs.
Graphical Abstract









Similar content being viewed by others
References
Sanaei MJ, Razi S, Pourbagheri-Sigaroodi A, Bashash D. The PI3K/Akt/mTOR pathway in lung cancer; oncogenic alterations, therapeutic opportunities, challenges, and a glance at the application of nanoparticles. Transl Oncol. 2022;18(January):101364. https://doi.org/10.1016/j.tranon.2022.101364.
Doumat G, et al. Drug repurposing in non-small cell lung carcinoma : old solutions for new problems. Curr Oncol. 2023;30:704–19. https://doi.org/10.3390/curroncol30010055.
Wu Y, Wang X, Li J, Ma H, Seshadri VDD. Benzo(A)Pyrene-induced lung cancer: chemo protective effect of coronarin D in Swiss albino mice. Appl Biochem Biotechnol. 2023;195(2):1122–35. https://doi.org/10.1007/s12010-022-04166-2.
Khan A, et al. Experimental and theoretical insights on chemopreventive effect of the liposomal thymoquinone against Benzo[a]pyrene-induced lung cancer in Swiss albino mice. J Inflamm Res. 2022;15(April):2263–80. https://doi.org/10.2147/JIR.S358632.
Guo B, et al. CD44-targeting hydrophobic phosphorylated gemcitabine prodrug nanotherapeutics augment lung cancer therapy. Acta Biomater. 2022;145:200–9. https://doi.org/10.1016/j.actbio.2022.04.016.
Wang H, et al. Aptamer-dendrimer bioconjugates for targeted delivery of miR-34a expressing plasmid and antitumor effects in non-small cell lung cancer cells. PLoS ONE. 2015;10(9):1–15. https://doi.org/10.1371/journal.pone.0139136.
Behl A, et al. Codelivery of gemcitabine and MUC1 inhibitor using PEG-PCL nanoparticles for breast cancer therapy. Mol Pharm. 2022;19(7):2429–40. https://doi.org/10.1021/acs.molpharmaceut.2c00175.
Hatami E, Nagesh PKB, Chauhan N, Jaggi M, Chauhan SC, Yallapu MM. In situ nanoparticle self-assembly for combination delivery of therapeutics to non-small cell lung cancer. ACS Appl Bio Mater. 2022;5(3):1104–19. https://doi.org/10.1021/acsabm.1c01158.
Kaur H, Kumar V, Kumar K, Rathor S, Kumari P, Singh J. Polymer particulates in drug delivery. Curr Pharm Des. 2016;22(19):2761–87. https://doi.org/10.2174/1381612822666160217125734.
Khot VM, Salunkhe AB, Pricl S, Bauer J, Thorat ND, Townley H. Nanomedicine-driven molecular targeting, drug delivery, and therapeutic approaches to cancer chemoresistance. Drug Discov Today. 2021;26(3):724–39. https://doi.org/10.1016/j.drudis.2020.12.016.
Kumar K, Rani V, Mishra M, Chawla R. New paradigm in combination therapy of siRNA with chemotherapeutic drugs for effective cancer therapy. Curr Res Pharmacol Drug Discov. 2022;3(April):100103. https://doi.org/10.1016/j.crphar.2022.100103.
Ding Y et al. RBC-hitchhiking chitosan nanoparticles loading methylprednisolone for lung-targeting delivery. J Control Release 2022;341(December 2021):702–715. https://doi.org/10.1016/j.jconrel.2021.12.018.
Zi Y, Yang K, He J, Wu Z, Liu J, Zhang W. Strategies to enhance drug delivery to solid tumors by harnessing the EPR effects and alternative targeting mechanisms. Adv Drug Deliv Rev. 2022;188(639): 114449. https://doi.org/10.1016/j.addr.2022.114449.
Kumar K, Chawla R. Nanocarriers-mediated therapeutics as a promising approach for treatment and diagnosis of lung cancer. J Drug Deliv Sci Technol. 2021;65(June):102677. https://doi.org/10.1016/j.jddst.2021.102677.
Vikas, Sahu HK, Mehata AK, Viswanadh MK, Priya V, Muthu MS. Dual-receptor-targeted nanomedicines: emerging trends and advances in lung cancer therapeutics. Nanomedicine 2022. https://doi.org/10.2217/nnm-2021-0470.
Dobie C, Skropeta D. Insights into the role of sialylation in cancer progression and metastasis. Br J Cancer. 2021;124(1):76–90. https://doi.org/10.1038/s41416-020-01126-7.
Gao Z et al. Abnormal sialylation and fucosylation of saliva glycoproteins: characteristics of lung cancer-specific biomarkers. Curr Res Pharmacol Drug Discov. 2022;3(December 2021):100079. https://doi.org/10.1016/j.crphar.2021.100079.
Munkley J, Scott E. Targeting aberrant sialylation to treat cancer. Medicines. 2019;6(4):102. https://doi.org/10.3390/medicines6040102.
Bose P, Jaiswal MK, Singh SK, Singh RK, Tiwari VK. Growing impact of sialic acid-containing glycans in future drug discovery. Carbohydr Res. 2023;527(March):108804. https://doi.org/10.1016/j.carres.2023.108804.
Berois N, Pittini A, Osinaga E. Targeting tumor glycans for cancer therapy : successes, limitations, and perspectives. 2022;14(645):2–42.
Vikas et al. Bioadhesive chitosan nanoparticles: dual targeting and pharmacokinetic aspects for advanced lung cancer treatment. Carbohydr Polym. 2021;274(March):118617. https://doi.org/10.1016/j.carbpol.2021.118617.
Vieira K, Luciana J, Siqueira N, Parize L. In vitro cytotoxic and antioxidant evaluation of quercetin loaded in ionic cross-linked chitosan nanoparticles. J Drug Deliv Sci Technol. 2022;74(February):1–9. https://doi.org/10.1016/j.jddst.2022.103561.
Huerta-Madroñal M, Espinosa-Cano E, Aguilar MR, Vazquez-Lasa B. Antiaging properties of antioxidant photoprotective polymeric nanoparticles loaded with coenzyme-Q10. Biomater Adv. 2022;145(November):2023. https://doi.org/10.1016/j.bioadv.2022.213247.
Ahmad N, et al. A comparative pulmonary pharmacokinetic study of budesonide using polymeric nanoparticles targeted to the lungs in treatment of asthma. Artif Cells Nanomed Biotechnol. 2020;48(1):749–62. https://doi.org/10.1080/21691401.2020.1748640.
Rana R, Rani S, Kumar V, Nakhate KT, Ajazuddin, Gupta U. Sialic acid conjugated chitosan nanoparticles: modulation to target tumour cells and therapeutic opportunities. AAPS PharmSciTech. 2022;23(1):1–16. https://doi.org/10.1208/s12249-021-02170-z.
Kumar K, Govind S, Mishra M, Kumar A, Chawla R. Dual targeting pH responsive chitosan nanoparticles for enhanced active cellular internalization of gemcitabine in non-small cell lung cancer. Int J Biol Macromol. 2023;249(July):126057. https://doi.org/10.1016/j.ijbiomac.2023.126057.
Chatterjee NS et al. Nano-encapsulation of curcumin in fish collagen grafted succinyl chitosan hydrogel accelerates wound healing process in experimental rats. Food Hydrocoll Heal. 2022;2(December 2021):100061. https://doi.org/10.1016/j.fhfh.2022.100061.
Beg S et al. Systematic development of solid lipid nanoparticles of abiraterone acetate with improved oral bioavailability and anticancer activity for prostate carcinoma treatment. 2022. https://doi.org/10.1021/acsomega.1c07254.
Zhang P et al. An intelligent hypoxia-relieving chitosan-based nanoplatform for enhanced targeted chemo-sonodynamic combination therapy on lung cancer. Carbohydr Polym. 2021;274(September):118655. https://doi.org/10.1016/j.carbpol.2021.118655.
Huang X et al. CKAP4 antibody-conjugated Si quantum dot micelles for targeted imaging of lung cancer. Nanoscale Res Lett. 2021;16(1). https://doi.org/10.1186/s11671-021-03575-2.
Lin X, Bai Y, Jiang Q. Precise fabrication of folic acid-targeted therapy on metformin encapsulated β-cyclodextrin nanomaterials for treatment and care of lung cancer. Process Biochem. 2022;118(39):74–83. https://doi.org/10.1016/j.procbio.2022.04.003.
Singh A, Xu J, Mattheolabakis G, Amiji M. “EGFR-targeted gelatin nanoparticles for systemic administration of gemcitabine in an orthotopic pancreatic cancer model”, Nanomedicine Nanotechnology. Biol Med. 2016;12(3):589–600. https://doi.org/10.1016/j.nano.2015.11.010.
Wang Y, Ma J, Qiu T, Tang M, Zhang X, Dong W. In vitro and in vivo combinatorial anticancer effects of oxaliplatin- and resveratrol-loaded N,O-carboxymethyl chitosan nanoparticles against colorectal cancer. Eur J Pharm Sci. 2021;163(October 2020):105864. https://doi.org/10.1016/j.ejps.2021.105864.
Onugwu AL, Attama AA, Nnamani PO, Onugwu SO, Onuigbo EB, Khutoryanskiy VV. Development and optimization of solid lipid nanoparticles coated with chitosan and poly(2-ethyl-2-oxazoline) for ocular drug delivery of ciprofloxacin. J Drug Deliv Sci Technol. 2022;74(February):103527. https://doi.org/10.1016/j.jddst.2022.103527.
Jadon RS et al. Efficient in vitro and in vivo docetaxel delivery mediated by pH-sensitive LPHNPs for effective breast cancer therapy. Colloids Surfaces B Biointerfaces. 2021; 203(July 2020):111760. https://doi.org/10.1016/j.colsurfb.2021.111760.
Anandakumar P, et al. Capsaicin inhibits benzo(a)pyrene-induced lung carcinogenesis in an in vivo mouse model. Inflamm Res. 2012;61(11):1169–75. https://doi.org/10.1007/s00011-012-0511-1.
Majumder D, Sarkar C, Debnath R, Tribedi P, Maiti D. Mechanistic insight into the synergism of IL-27 and IL-28B in regulation of benzo(a)pyrene-induced lung carcinogenesis associated ROS/NF-κB/NLRP3 crosstalk. Chem Biol Interact. 2022;354(January):109807. https://doi.org/10.1016/j.cbi.2022.109807.
Yan C, Shi W, Gu J, Lee RJ, Zhang Y. Design of a novel nucleus-targeted NLS-KALA-SA nanocarrier to delivery poorly water-soluble anti-tumor drug for lung cancer treatment. J Pharm Sci. 2021;110(6):2432–41. https://doi.org/10.1016/j.xphs.2020.12.034.
Essa ML, Elashkar AA, Hanafy NAN, Saied EM, El-Kemary M. Dual targeting nanoparticles based on hyaluronic and folic acids as a promising delivery system of the encapsulated 4-Methylumbelliferone (4-MU) against invasiveness of lung cancer in vivo and in vitro. Int J Biol Macromol. 2022;206(February):467–80. https://doi.org/10.1016/j.ijbiomac.2022.02.095.
Kim J et al. The safe and effective intraperitoneal chemotherapy with cathepsin B-specific doxorubicin prodrug nanoparticles in ovarian cancer with peritoneal carcinomatosis. Biomaterials. 2021;279(July):121189. https://doi.org/10.1016/j.biomaterials.2021.121189.
Wei D, et al. Stroma-targeted nanoparticles that remodel stromal alignment to enhance drug delivery and improve the antitumor efficacy of Nab-paclitaxel in pancreatic ductal adenocarcinoma models. Nano Today. 2022;45: 101533. https://doi.org/10.1016/j.nantod.2022.101533.
Kumar K, Kumar V, Lal J, Kaur H, Singh J. A simple 2D composite image analysis technique for the crystal growth study of L-ascorbic acid. Microsc Res Tech. 2017;80(6):615–26. https://doi.org/10.1002/jemt.22838.
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–24. https://doi.org/10.1038/s41573-020-0090-8.
Liao J, Song Y, Liu C, Li D, Zheng H, Lu B. Dual-drug delivery based charge-conversional polymeric micelles for enhanced cellular uptake and combination therapy. Polym Chem. 2019;10(43):5879–93. https://doi.org/10.1039/c9py01105f.
Hanafy NAN, Salim EI, Mahfouz ME, Eltonouby EA, Hamed IH. Fabrication and characterization of bee pollen extract nanoparticles : their potential in combination therapy against human A549 lung cancer cells. Food Hydrocoll Heal. 2023;3(100110). https://doi.org/10.1016/j.fhfh.2022.100110.
Weston A et al. The influence of ions on the lubricative abilities of mucin and the role of sialic acids. Colloids Surfaces B Biointerfaces. 2023; 227(November 2022):113327. https://doi.org/10.1016/j.colsurfb.2023.113327.
Guan M et al. N-trimethyl chitosan nanoparticle-encapsulated lactosyl-norcantharidin for liver cancer therapy with high targeting efficacy. Nanomed Nanotechnol Biol Med. 2012;8(7):1172–1181. https://doi.org/10.1016/j.nano.2012.01.009.
Vanza JD, Shah DM, Patel RB, Patel MR. Afatinib liposomal dry powder inhaler: Targeted pulmonary delivery of EGFR inhibitor for the management of lung cancer. J Drug Deliv Sci Technol. 2022;74(March):103506. https://doi.org/10.1016/j.jddst.2022.103506.
Shakiba S, Astete CE, Cueto R, Rodrigues DF, Sabliov CM, Louie SM. Asymmetric flow field-flow fractionation (AF4) with fluorescence and multi-detector analysis for direct, real-time, size-resolved measurements of drug release from polymeric nanoparticles. J Control Release. 2021;338(March):410–21. https://doi.org/10.1016/j.jconrel.2021.08.041.
Milligan JJ, Saha S. A nanoparticle’s journey to the tumor: strategies to overcome first-pass metabolism and their limitations. Cancers (Basel). 2022;14(7). https://doi.org/10.3390/cancers14071741.
Acknowledgements
The authors express their gratitude to the Ministry of Human Resource and Development (MHRD)-India for providing a Teaching Assistantship. Furthermore, the authors would like to acknowledge CIF-IIT BHU for their assistance in conducting the instrumental characterization of formulations.
Author information
Authors and Affiliations
Contributions
Krishan Kumar: conceptualization, project administration, writing — original draft, review, and editing, methodology, formal analysis, resources, investigation, software; Rinki Verma: resources; Manjit: resources, validation, data curation; Priya: writing — review and editing; Mohini Mishra: visualization; Varsha Rani: data curation; Ruchi Chawla: supervision, visualization, writing — review and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, K., Verma, R., Manjit et al. In Vivo Cancer Microenvironment Responsive Glycan Receptor-Targeted Nanoparticles for Gemcitabine Delivery to Benzo[a]pyrene-Induced Lung Cancer Model. AAPS PharmSciTech 25, 2 (2024). https://doi.org/10.1208/s12249-023-02714-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02714-5