Abstract
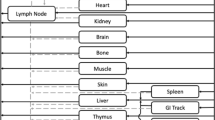
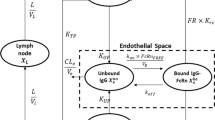
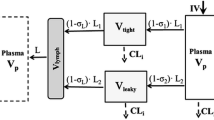
Antibody-based therapeutics have recently gained keen attention for the treatment of pulmonary indications. However, systemically administered antibody exposure in the lungs needs to be better understood and remains a topic of interest. In this study, we evaluated the exposure of two different uPAR (urokinase-type plasminogen activator receptor) targeting full-length monoclonal IgGs in plasma and lung epithelial lining fluid (ELF) of mice after IP and IV administration. Antibody AK17 exhibited linear pharmacokinetics (PK) in plasma and ELF at 3 and 30 mg/kg single IV dose. The average plasma and ELF half-lives for AK17 and AK21 ranged between ~321–411 h and ~230–345 h, respectively, indicating sustained systemic and lung exposure of antibodies. The average ELF to the plasma concentration ratio of antibodies was ~0.01 and ~0.03 with IP and IV dosing, respectively, over 2 weeks post single dose. We simultaneously characterized plasma and ELF PK of antibody in mice by developing a minimal lung PBPK model for antibody. This model reasonably captured the plasma and ELF PK data while estimating three parameters. The model accounts for the convective transport of antibody into the tissues via blood and lymph flow. FcRn-mediated transcytosis was incorporated into the model for antibody distribution across the lung epithelial barrier. This model serves as a platform to predict the pulmonary PK of systemically administered antibodies and to support optimal dose selection for desired exposure in the lungs as the site of action.





Similar content being viewed by others
References
Roth-Walter F, Adcock IM, Benito-Villalvilla C, Bianchini R, Bjermer L, Caramori G, et al. Comparing biologicals and small molecule drug therapies for chronic respiratory diseases: an EAACI Taskforce on Immunopharmacology position paper. Allergy. 2019;74:432–48. Ava ilable from: https://onlinelibrary.wiley.com/doi/10.1111/all.13642
Bitonti AJ, Dumont JA. Pulmonary administration of therapeutic proteins using an immunoglobulin transport pathway. Adv Drug Deliv Rev. 2006;58:1106–18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16997417
Liang W, Pan HW, Vllasaliu D, Lam JKW. Pulmonary delivery of biological drugs. Pharmaceutics. 2020:12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33114726
Johannson KA, Chaudhuri N, Adegunsoye A, Wolters PJ. Treatment of fibrotic interstitial lung disease: current approaches and future directions. Lancet. 2021;398:1450–60. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673621018262
Gao W, Li L, Wang Y, Zhang S, Adcock IM, Barnes PJ, et al. Bronchial epithelial cells: the key effector cells in the pathogenesis of chronic obstructive pulmonary disease? Respirology. 2015;20:722–9. Available from: https://onlinelibrary.wiley.com/doi/10.1111/resp.12542
Parray HA, Shukla S, Perween R, Khatri R, Shrivastava T, Singh V, et al. Inhalation monoclonal antibody therapy: a new way to treat and manage respiratory infections. Appl Microbiol Biotechnol. 2021;105:6315–32. Available from: https://link.springer.com/10.1007/s00253-021-11488-4
Mu R, Yuan J, Huang Y, Meissen JK, Mou S, Liang M, et al. Bioanalytical methods and strategic perspectives addressing the rising complexity of novel bioconjugates and delivery routes for biotherapeutics. BioDrugs. 2022;36:181–96. Available from: https://link.springer.com/10.1007/s40259-022-00518-w
Leth JM, Ploug M. Targeting the urokinase-type plasminogen activator receptor (uPAR) in human diseases with a view to non-invasive imaging and therapeutic intervention. Front Cell Dev Biol. 2021:9. Available from: https://www.frontiersin.org/articles/10.3389/fcell.2021.732015/full
Marudamuthu AS, Bhandary YP, Shetty SK, Fu J, Sathish V, Prakash Y, et al. Role of the urokinase-fibrinolytic system in epithelial–mesenchymal transition during lung injury. Am J Pathol. 2015;185:55–68. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002944014005562
Myzithras M, Lin S, Radden L, Hess Kenny C, Cai Z, MacDonald A, et al. Development of novel ultra-sensitive IL-11 target engagement assays to support mechanistic PK/PD modeling for an anti-IL-11 antibody therapeutic. MAbs. 2022;14:2104153. Available from: http://www.ncbi.nlm.nih.gov/pubmed/35916739
Wurm M, Schaaf O, Reutner K, Ganesan R, Mostböck S, Pelster C, et al. A novel antagonistic CD73 antibody for inhibition of the immunosuppressive adenosine pathway. Mol Cancer Ther. 2021;20:2250–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34482286
Wu HH, Ralph K-L, Sepuldeva E, Hansen G, Li H, Huang Z-F, et al. An optimally designed anti-human CD40 antibody with potent B cell suppression for the treatment of autoimmune diseases. Int J Pharm. 2021;609:121162. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34624444
Fröhlich E, Mercuri A, Wu S, Salar-Behzadi S. Measurements of deposition, lung surface area and lung fluid for simulation of inhaled compounds. Front Pharmacol. 2016;7:181 http://www.ncbi.nlm.nih.gov/pubmed/27445817.
Jia J, Yin Z, Zhang X, Li H, Meng D, Liu Q, et al. Feasibility studies of nebulized SARS-CoV-2 neutralizing antibody in mice and cynomolgus monkeys. Pharm Res. 2022; Available from: http://www.ncbi.nlm.nih.gov/pubmed/35882740
Shah DK, Betts AM. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn. 2012;39:67–86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22143261
Garg A, Balthasar JP. Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn. 2007;34:687–709. Available from: http://link.springer.com/10.1007/s10928-007-9065-1
Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol. 1992;6:235–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1540387
Sécher T, Guilleminault L, Reckamp K, Amanam I, Plantier L, Heuzé-Vourc’h N. Therapeutic antibodies: a new era in the treatment of respiratory diseases? Pharmacol Ther. 2018;189:149–72. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0163725818300779
Sifniotis V, Cruz E, Eroglu B, Kayser V. Current advancements in addressing key challenges of therapeutic antibody design, manufacture, and formulation. Antibodies. 2019;8:36. Available from: https://www.mdpi.com/2073-4468/8/2/36
Li Z, Krippendorff B-F, Sharma S, Walz AC, Lavé T, Shah DK. Influence of molecular size on tissue distribution of antibody fragments. MAbs. 2016;8:113–9. Available from: https://www.tandfonline.com/doi/full/10.1080/19420862.2015.1111497
Shah DK, Betts AM. Antibody biodistribution coefficients. MAbs. 2013;5:297–305. Available from: http://www.tandfonline.com/doi/abs/10.4161/mabs.23684
Zhai B-T, Tian H, Sun J, Zou J-B, Zhang X-F, Cheng J-X, et al. Urokinase-type plasminogen activator receptor (uPAR) as a therapeutic target in cancer. J Transl Med. 2022;20:135. Available from: https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-022-03329-3
Mahmood N, Mihalcioiu C, Rabbani SA. Multifaceted role of the urokinase-type plasminogen activator (uPA) and its receptor (uPAR): diagnostic, prognostic, and therapeutic applications. Front Oncol. 2018:8. Available from: http://journal.frontiersin.org/article/10.3389/fonc.2018.00024/full
Sakagami M, Omidi Y, Campbell L, Kandalaft LE, Morris CJ, Barar J, et al. Expression and transport functionality of FcRn within rat alveolar epithelium: a study in primary cell culture and in the isolated perfused lung. Pharm Res. 2006;23:270–9. Available from: http://link.springer.com/10.1007/s11095-005-9226-0
Kim K-J, Malik AB. Protein transport across the lung epithelial barrier. Am J Physiol Lung Cell Mol Physiol. 2003;284:L247–59. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12533309
Kim K-J, Fandy TE, Lee VHL, Ann DK, Borok Z, Crandall ED. Net absorption of IgG via FcRn-mediated transcytosis across rat alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2004;287:L616–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15169676
Dall’Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem. 2006;281:23514–24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16793771
Lavoisier A, Schlaeppi J-M. Early developability screen of therapeutic antibody candidates using Taylor dispersion analysis and UV area imaging detection. MAbs. 2015;7:77–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25514497
Matsukawa Y, Lee VHL, Crandall ED, Kim K-J. Size-dependent dextran transport across rat alveolar epithelial cell monolayers. J Pharm Sci. 1997;86:305–9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S002235491550261X
Sockolosky JT, Szoka FC. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv Drug Deliv Rev. 2015;91:109–24. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0169409X15000162
Gaohua L, Wedagedera J, Small BG, Almond L, Romero K, Hermann D, et al. Development of a multicompartment permeability-limited lung PBPK model and its application in predicting pulmonary pharmacokinetics of antituberculosis drugs. CPT pharmacometrics Syst Pharmacol. 2015;4:605–13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26535161
Author information
Authors and Affiliations
Contributions
SS and AL: In vivo PK study design and experimental work. KP: anti-uPAR antibody generation and characterization. SS, HYC, and JW: antibody minimal lung PBPK model development. SS: writing original manuscript. HYC, JW, and SH: review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Philip Kwok, Francesca Buttini, Jenny Lam and Vivek Gupta
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 135 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, S., Leonard, A., Phoenix, K. et al. Systemically Administered Anti-uPAR Antibody Plasma and Lung ELF Pharmacokinetics Characterized by Minimal Lung PBPK Model. AAPS PharmSciTech 24, 236 (2023). https://doi.org/10.1208/s12249-023-02689-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02689-3