Abstract
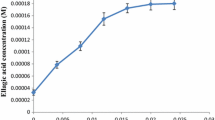
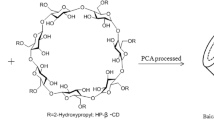
The main components of Caesalpinia sappan L. (CS) are brazilin and brazilein, which show high potential in pharmacologic applications. However, these have been drastically limited by the poor water solubility and stability. The present study investigates the formation of inclusion complexes F1, F2, and F3 between CS and β-cyclodextrin (βCD), hydroxypropyl-β-cyclodextrin (HPβCD), and methyl-β-cyclodextrin (MβCD), respectively. These complexes were characterized by Fourier transform infrared spectroscopy (FT-IR). The results showed that the highest encapsulation efficiency and loading capacity of CS extract were 44.24% and 9.67%, respectively. The solubility and stability of CS extract were significantly increased through complexation in phase solubility and stability studies. The complexes F1–F3 showed mainly significant antibacterial activities on gram-positive bacteria pathogens causing mastitis. Moreover, the expression levels of COX-2 and iNOS were significantly decreased in LPS-induced inflammatory cells at concentrations of 50 and 100 µg/mL. In addition, treatment of complex F3 (CS/MβCD) in bovine endothelial cells remarkably increased the chemokine gene expression of CXCL3 and CXCL8, which were responsible for immune cell recruitment (9.92 to 11.17 and 8.23 to 9.51–fold relative to that of the LPS-treated group, respectively). This study provides a complete characterization of inclusion complexes between CS extract and βCD, HPβCD, and MβCD for the first time, highlighting the impact of complex formation on the pharmacologic activities of bovine mastitis.
Graphical Abstract







Similar content being viewed by others
References
Kibebew K. Bovine mastitis: a review of causes and epidemiological point of view. J Bio Agr Healthcare. 2017;7(2):1–14.
Thompson-Crispi K, Atalla H, Miglior F, Mallard BA. Bovine mastitis: frontiers in immunogenetics. Front Immunol. 2014;5(493):1–10.
Cheng WN, Han SG. Bovine mastitis: risk factors, therapeutic strategies, and alternative treatments-a review. Asian-Australas J Anim Sci. 2020;33(11):1699–713.
Boonyayatra S. Treatment of bovine mastitis during lactating period. Chiang Mai Veterinary J. 2012;10(2):89–109.
Rossiter SE, Fletcher MH, Wuest WM. Natural products as platforms to overcome antibiotic resistance. Chem Rev. 2017;117(19):12415–74.
Su T, Qiu Y, Hua X, Ye B, Luo H, Liu D, et al. Novel opportunity to reverse antibiotic resistance: to explore traditional Chinese medicine with potential activity against antibiotics-resistance bacteria. Front Microbiol. 2020;11:1–11.
Zhao H, Bai H, Wang Y, Li W, Koike K. A new homoisoflavan from Caesalpinia sappan. J Nat Med. 2008;62(3):325–7.
Toegel S, Wu SQ, Otero M, Goldring MB, Leelapornpisid P, Chiari C, et al. Caesalpinina sappan extract inhibits IL 1b-mediated overexpression of matrix metalloproteinases in human chondrocytes. Genes Nutri. 2012;7(2):312–8.
Sireeratawong S, Piyabhan P, Singhalak T, Wongkrajang Y, Temsiririrkkul R, Punsrirat J, et al. Toxicity evaluation of sappan wood extract in rats. J Med Assoc Thai. 2010;93(7):50–7.
Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. Beijing: Medical Science and Technology Press; 2015.
China Pharmacopoeia Commission. The Chinese pharmacopoeia. Beijing: China Medical Science Press; 2010. p. 153.
Zhao HX, Bai H, Wang YS. The NMR characterization of Caesalpinia sappan phenolic compounds. Qilu Pharm Affairs. 2007;26(7):417–9.
Li Q, Wang RP, Zou X. Research progress on chemical constituents and antitumor Caesalpinia sappan. J Tradit Chin Med Univ Hunan. 2012;32(4):76–8.
Badami S, Moorkoth S, Suresh B. Caesalpinia sappan-a medicinal and dye yielding plant. Indian J Nat Prod Res. 2004;3(2):75–82.
Jin SK, Ha SR, Choi JS. Effect of Caesalpinia sappan L extract on physico-chemical properties of emulsion-type pork sausage during cold storage. Meat Sci. 2015;110:245–52.
Washiyama M, Sasaki Y, Hosokawa T, Nagumo S. Anti-inflammatory constituents of Sappan lignum. Biol Pharma Bull. 2009;32(5):941–4.
Handayani S, Susidarti RA, Jenie RI, Meiyanto E. Two active compounds from Caesalpinia sappan L. In combination with cisplatin synergistically induce apoptosis and cell cycle arrest on WiDr cells. Adv Pharm Bull. 2017;7(3):375–80.
Cuong TD, Hung TM, Kim JC, Kim EH, Woo MH, Choi JH, et al. Phenolic compounds from Caesalpinia sappan heartwood and their anti-inflammatory activity. J Nat Prod. 2012;75:2069–75.
Sasaki Y, Hosokawa T, Nagai M, Nagumo S. In vitro study for inhibition of NO production about constituents of Sappan lignum. Biol Pharma Bull. 2007;30(1):193–6.
Nirmal NP, Rajput MS, Prasad RGSV, Ahmad M. Brazilin from Caesalpinia sappan heartwood and its pharmacological activities: a review. Asian Pac J Trop Med. 2015;8(6):421–30.
Xu HX, Lee SF. The antibacterial principle of Caesalpinia sappan. Phytother Res. 2004;18(8):647–51.
Li CX, Si CP, Gao HJ, Ming JK, Fu J, Sun M. Experimental study Caesalpinia sappan aqueous extract to induce immune tolerance in allogeneic skin transplantation. Chin J Cell Mol Immunol. 2011;27(2):186–9.
Badami S, Moorkoth S, Rai SR, Kannan E, Bhojraj S. Antioxidant activity of Caesalpinia sappan heartwood. Biol Pharm Bull. 2003;26(11):1534–7.
Niu Y, Wang S, Li C, Wang J, Liu Z, Kang W. Effective compounds from Caesalpinia sappan L. on the tyrosinase in vitro and in vivo. Nat Prod Commun. 2020;15(4):1–8.
Xu JG, Guo ST, Qiao LJ. The effect of Caesalpinia sappan L. extract on tumor. Cancer Res Clin. 2006;18(11):726–8.
Khil LY, Han SS, Kim SG, Chang TS, Jeon SD, So DS, et al. Effects of brazilin on GLUT4 recruitment in isolated rat epididymal adipocytes. Biochem Pharmacol. 1999;58(11):1705–12.
Khil LY, Moon CK. Hydrogen peroxide mediates brazilin-induced glucose transport in adipocytes. J Appl Pharmacol. 2004;12(4):228–34.
Watkins R, Wu L, Zhang C, Davis RM, Xu B. Natural product-based nanomedicine: recent advances and issues. Int J Nanomed. 2015;10:6055–74.
Wüpper S, Lüersen K, Rimbach R. Cyclodextrins, natural compounds, and plant bioactives a nutritional perspective. Biomolecules. 2021;11:401–21.
Singh M, Sharma R, Banerjee UC. Biotechnological applications of cyclodextrins. Biotechnol Adv. 2002;20(5–6):341–59.
Loftsson T, Jarho P, Másson M, Järvinen T. Cyclodextrins in drug delivery. Expert Opin Drug Deliv. 2005;2(2):335–51.
Poulson BG, Alsulami QA, Sharfalddin A, El Agammy EF, Mouffouk F, Emwas AH, et al. Cyclodextrins: structural, chemical, and physical properties, and applications. Polysaccharides. 2022;3:1–31.
Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998;98:1743–54.
Connors KA. The Stability of cyclodextrin complexes in solution. Chem Rev. 1997;97:1325–58.
Saenger W, Jacob J, Gessler K, Steiner T, Hoffmann D, Sanbe H, et al. Structures of the common cyclodextrins and their larger analogues beyond the doughnut. Chem Rev. 1998;98:1787–802.
Gidwani B, Vyas A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. Bio Med Res Int. 2015;2015:198268.
Aloisioa C, Longhi M. Diloxanide furoate binary complexes with β-, methyl-β- and hydroxypropyl-β-cyclodextrins: inclusion mode, characterization in solution and in solid state and in-vitro dissolution studies. Pharm Dev Technol. 2017;23(7):723–31.
Sandilya AA, Natarajan U, Priya MH. Molecular view into the cyclodextrin cavity: structure and hydration. ACS Omega. 2020;40(5):25655–67.
Jug M. Cyclodextrin-based drug delivery systems. nanomaterials for clinical applications. 2020;29–68.
Crini G, Fourmentin S, Fenyvesi E, Torri G, Fourmentin M, Morin-Crini N. Cyclodextrins, from molecules to applications. Environmental Chem Lett. 2018;16:1361–75.
Arsiningtyas IS. Antioxidant profile of heartwood and sapwood of Caesalpinia sappan L tree’s part grown in Imogiri Nature Preserve, Yogyakarta. IOP Conf Series Earth Environ Sci. 2021;810(1):012040.
Khamsita R, Hermawan A, Putri DDP, Meiyanto E. Ethanolic extract of Secang (Caesalpinia sappan L.) wood performs as chemosensitizing agent through apoptotic induction on breast cancer MCF-7 Cells. Ind J Cancer Chemo Prev. 2012;3(3):444–9.
Loescher CM, Morton DW, Razic S, Agatonovic-Kustrin S. High performance thin layer chromatography (HPTLC) and high performance liquid chromatography (HPLC) for the qualitative and quantitative analysis of Calendula officinalis–advantages and limitations. J Pharm Biomed Anal. 2014;98:52–9.
Moricz AM, Lapat V, Morlock GE, Ott PG. High-performance thin-layer chromatography hyphenated to high-performance liquid chromatography-diode array detection-mass spectrometry for characterization of coeluting isomers. Talanta. 2020;219:121306–11.
He J, Guo F, Lin L, Chen H, Chen J, Cheng Y, et al. Investigating the oxyresveratrol β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin complexes: the effects on oxyresveratrol solution, stability, and antibrowning ability on fresh grape juice. LWT-Food Sci Tech. 2018.
Das S, Gazdag Z, Szente L, Meggyes M, Horváth G, Lemli B, et al. Antioxidant and antimicrobial properties of randomly methylated β cyclodextrin-captured essential oils, Food Chem. 2018.
Chaisri W, Hennink WE, Ampasavate C, Okonogi S. Cephalexin microspheres for dairy mastitis: effect of preparation method and surfactant type on physicochemical properties of the microspheres. AAPS Pharm Sci Tech. 2010;11(2):945–51.
Najlah M, Parveen I, Alhnan MA, Ahmed W, Faheem A, Phoenix DA, et al. The effects of suspension particle size on the performance of air-jet, ultrasonic and vibrating-mesh nebulisers. Int J Pharm. 2014;461:234–41.
Higuchi T, Connors KA. Phase-solubility techniques. In Advances in Analytical Chemistry and Instrumentation. Reilly CN, editor. Wiley-Interscience; 1965. 4. p. 117−212.
Siva S, Li C, Cui H, Lin L. Encompassment of isoeugenol in 2-hydroxypropyl-β-cyclodextrin using ultrasonication: characterization, antioxidant and antibacterial activities. J Mol Liquids. 2019;296:111777.
Siva S, Lib C, Cuia H, Meenatchic V, Lin L. Encapsulation of essential oil components with methyl-β-cyclodextrin using ultrasonication: solubility, characterization, DPPH and antibacterial assay. Ultrasonics - Sonochemistry. 2020;64:104997.
Yang LJ, Chang Q, Zhou SY, Yang YH, Xia FT, Chen W, et al. Host–guest interaction between brazilin and hydroxypropyl-β-cyclodextrin: preparation, inclusion mode, molecular modelling and characterization. Dyes Pigments. 2018;150:193–201.
Crupi V, Ficarra R, Guardo M, Majolino D, Stancanelli R, Venuti V. UV–vis and FTIR–ATR spectroscopic techniques to study the inclusion complexes of genistein with B-cyclodextrins. J Pharm Biomed Anal. 2007;44:110–7.
ICH harmonised tripartite guideline, stability testing of new drug substances and products Q1A(R2), Current Step 4 version, dated 6 February 2003.
ASEAN guidelines on stability study of drug product (R1), February 2005, Revision 9th ACCSQ-PPWG, Meeting; Philippines.
Clinical and Laboratory Standards Institue (CLSI). Performance standards for antimicobial susceptibility testing: twenty-fifth informational supplement; 2015;35(3): 1−236.
Bae IK, Min HY, Han AR, Seo EK, Lee SK. Suppression of lipopolysacchride-induced expression of inducible nitric oxide synthase by brazilin in RAW 264.7 macrophage cells. Eur J Pharmacol. 2005;513(3):237–42.
Panya A, Pundith H, Thongyim S, Kaewkod T, Chitov T, Bovonsombut S, et al. Antibiotic-antiapoptotic dual function of clinacanthus nutans (Burm. F.) lindau leaf extracts against bovine mastitis. Antibiotics. 2020;9:429.
Thongyim S, Chiangchin S, Pandith H, Tragoolpua Y, Jangsutthivorawat S, Panya A. Anti-inflammatory activity of glyceryl 1,3-distearate identified from Clinacanthus nutans extract against bovine mastitis pathogens. Antibiotics. 2023;12:549.
Hemthanon T, Ungcharoenwiwat P. Antibacterial activity, stability, and hemolytic activity of heartwood extract from Caesalpinia sappan for application on nonwoven fabric. Elect J Biotechnol. 2022;55:9–17.
Ngamwonglumlert L, Devahastin S, Chiewchan N, Raghavan GSV. Color and molecular structure alterations of brazilein extracted from Caesalpinia sappan L under different pH and heating conditions. Scientific Reports. 2020;10(1):12386–95.
Gomes LMM, Petito N, Costa VG, Falcão DQ, de Lima Araújo KG. Inclusion complexes of red bell pepper pigments with β-cyclodextrin: preparation, characterisation and application as natural colorant in yogurt. Food Chem. 2014;148:428–36.
Trenkenschuh E, Friess W. Freeze-drying of nanoparticles: how to overcome colloidal instability by formulation and process optimization. Eur J Pharm Biopharm. 2021;165:345–60.
Badr-Eldin SM, Elkheshen SA, Ghorab MM. Inclusion complexes of tadalafil with natural and chemically modified β-cyclodextrins. I: Preparation and in-vitro evaluation. Eur J Pharm Biopharm. 2008;70:819–27.
Kalogeropoulos N, Konteles S, Mourtzinos I, Troullidou E, Chiou A, Karathanos VT. Encapsulation of complex extracts in β-cyclodextrin: an application to propolis ethanolic extract. J Microencapsulation. 2009;26(7):603–13.
Mourtzinos I, Salta F, Yannakopoulou K, Chiou A, Karathanos VT. Encapsulation of olive leaf extract in β-cyclodextrin. J Agric Food Chem. 2007;55:8088–94.
Santos PS, Souza LM, Araujo TSL, Medeiros JVR, Nunes SCC, Carvalho RA, et al. Methyl-β-cyclodextrin inclusion complex with β-caryophyllene: preparation, characterization, and improvement of pharmacological activities. ACS Omega. 2017;2:9080–94.
Fenyvesi F, Nguyen TLP, Haimhoffer A, Rusznyák A, Vasvári G, Bácskay I, et al. Cyclodextrin complexation improves the solubility and Caco-2 permeability of chrysin. Materials. 2020;13:3618–29.
Arima H, Yunomae K, Miyake K, Irie T, Hirayama F, Uekama K. Comparative studies of the enhancing effects of cyclodextrins on the solubility and oral bioavailability of tacrolimus in rats. J Pharm Sci. 2001;90:690–701.
Mura P. Analytical techniques for characterization of cyclodextrin complexes in aqueous solution: a review. J Pharm Biomed Anal. 2014;101:238–50.
Liu B, Li W, Zhao J, Liu Y, Zhu X, Liang G. Physicochemical characterisation of the supramolecular structure of luteolin/cyclodextrin inclusion complex. Food Chem. 2013;141:900–6.
Pattananandecha T, Apichai S, Julsrigival J, Ogata F, Kawasaki N, Saenjum C. Antibacterial activity against foodborne pathogens and inhibitory effect on anti-inflammatory mediators’ production of brazilin-enriched extract from Caesalpinia sappan Linn. Plants (Basel). 2022;11(13):1698.
Puttipan R, Chansakaow S, Khongkhunthian S, Okonogi S. Caesalpinia sappan: a promising natural source of antimicrobial agent for inhibition of cariogenic bacteria. Drug Dis Therapeutics. 2018;12(4):197–205.
Nirmal NP, Panichayupakaranant P. Antioxidant, antibacterial, and anti-inflammatory activities of standardized brazilin-rich Caesalpinia sappan extract. Pharm Biol. 2015;53(9):1339–43.
Xu HX, Lee SF. The antibacterial principle of Caesalpinia sappan. Phytother Res. 2004;18(8):647–51.
Liu Y, Chen W, Zheng F, Yu H, Wei K. Xanthatin alleviates LPS-induced inflammatory response in RAW264.7 macrophages by inhibiting NF-κB MAPK and STATs activation. Molecules. 2022;27:4603–17.
Xiong H, Cheng T, Zhang X, Zhang X. Effects of taraxasterol on iNOS and COX-2 expression in LPS-induced RAW 264.7 macrophages. J Ethnopharmacol. 2014;155:753–7.
Wu SQ, Otero M, Unger FM, Goldring MB, Phrutivorapongkul A, Chiari C, Toegel S. Anti-inflammatory activity of an ethanolic Caesalpinia sappan extract in human chondrocytes and macrophages. J Ethnopharmacol. 2011;138(2):364–72.
Tewtrakul S, Tungcharoen P, Sudsai T, Karalai C, Ponglimanont C, Yodsaoue O. Antiinflammatory and wound healing effects of Caesalpinia sappan L. Phytotherapy Res. 2015;29(6):850–6.
Min BS, Cuong TD. Phenolic compounds from Caesalpinia sappan and their inhibitory effects on LPS-induced NO production in RAW264.7 ells. Nat Prod Sci. 2013;19(3):201–5.
Hoskina RT, Xiong J, Esposito DA, Lila MA. Blueberry polyphenol-protein food ingredients: the impact of spray drying on the in vitro antioxidant activity, anti-inflammatory markers, glucose metabolism and fibroblast migration. Food Chem. 2019;280:187–94.
Macías-Cortés E, Gallegos-Infante JA, Rocha-Guzmán NE, Moreno-Jiménez MR, Villanueva-Fierro I, Ochoa-Martínez LA, et al. Spray drying conditions of antioxidant and anti-inflammatory polyphenols in microcapsules of ultrasound assisted extract of salvilla (Buddleja scordioides Kunth). ACS Food Sci Technol. 2022;2:1574–85.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: effects on drug permeation through biological membranes. J Pharm Pharmacol. 2011;63:1119–35.
Stella VJ, He Q. Cyclodextrins. Toxicol Pathol. 2008;36:30–42.
Zhao Q, Temsamani J, Iadarola PL, Agrawal S. Modulation of oligonucleotide-induced immune stimulation by cyclodextrin analogs. Biochem Pharmacol. 1996;52(10):1537–44.
Geisshüsler S, Schineis P, Langer L, Wäckerle-Men Y, Leroux JC, Halin C, et al. Amphiphilic cyclodextrin-based nanoparticulate vaccines can trigger T-Cell immune responses. Adv Nano Biomed Res. 2021;2(4):2100082–94.
Islam MdA, Takagi M, Fukuyama K, Komatsu R, Albarracin L, Nochi T, et al. Transcriptome analysis of the inflammatory responses of bovine mammary epithelial cells: exploring immunomodulatory target genes for bovine mastitis. Pathogens. 2020;9(3):200.
Ryman VE, Packiriswamy N, Sordillo LM. Role of endothelial cells in bovine mammary gland health and disease. Published online by Cambridge University Press: 25 August 2015.
Acknowledgements
We sincerely thank the Department of Pharmaceutical Science, Faculty of Pharmacy, Chiang Mai University, Thailand, for supporting analytical instruments and support of the work in this manuscript. We also thank Asst. Prof. Dr. Thararat Chitov, Department of Biology Faculty of Science, Chiang Mai University, for the heartwood materials of C. sappan.
Funding
This work was supported by the National Research Council of Thailand (NRCT) 2019.
Author information
Authors and Affiliations
Contributions
Conceptualization and funding acquisition: RK, WC; method, design experiments, and investigation: WC, PS, KT, HP, AP; writing—original draft writing: PS, WC, HP, AP; writing—review and editing: RK; supervision and analysis of data: RK, SP, WS, SO. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaisri, W., Suebsakwong, P., Pandith, H. et al. Effects of Encapsulation of Caesalpinia sappan L. with Cyclodextrins for Bovine Mastitis. AAPS PharmSciTech 24, 230 (2023). https://doi.org/10.1208/s12249-023-02687-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02687-5