Abstract
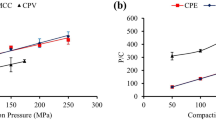
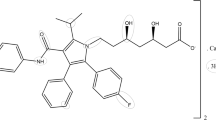
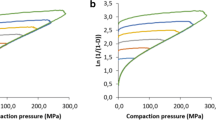
The physics of tablets mixtures has gained much attention lately. The purpose of this work is to evaluate the compaction properties of Kollidon® SR (KSR) in the presence of different excipients such as Microcrystalline cellulose (MCC), Monohydrous lactose (MH Lactose), Poly (vinyl acetate) (PVA100), and a water-soluble drug Diclofenac sodium (DNa) to prepare once daily formulation. Tablets were prepared using direct compression and were compressed into flat-faced tablets using hydraulic press at various pressures. The combination of MCC and KSR in the tablets showed reduced porosity, and almost constant low Py values as KSR levels increased; also, KSR-DNa tablets had higher percentage porosity and crushing strength values than KSR-MH Lactose tablets. The crushing strengths of KSR-MCC tablets were larger than those of KSR-DNa tablets. Ternary mixture tablets comprised of KSR-MCC-DNa showed decreased porosities and low Py values as the percentage of KSR increased especially at high compression pressures but had higher crushing strengths compared to KSR-DNa or MCC-DNa binary tablets. KSR-MH Lactose-DNa ternary tablets experienced lower porosities and crushing strengths compared to KSR-MCC-DNa tablets. Quaternary tablets of KSR-PVA100-MCC-DNa showed lower porosity and Py values than quaternary tablets obtained using similar proportion of MH Lactose instead of MCC. In conclusion, optimum quaternary tablets were obtained with optimum crushing strengths, relatively low Py, and moderate percentage porosities among all prepared quaternary tablets. The drug release of the optimum quaternary tablets demonstrated similar in vitro release profile compared to that of the marketed product with a mechanism of release that follows Korsmeyer-Peppas model.





Similar content being viewed by others
Data Availability
Data are available at the corresponding author and ready to be provided at any time upon request.
Change history
30 November 2023
A Correction to this paper has been published: https://doi.org/10.1208/s12249-023-02707-4
References
Müller-Goymann C, Schubert R, Bauer K-H, Frömming K-H, Führer C, Müller-Goymann C, et al. Bauer/Frömming/Führer Pharmazeutische Technologie: Mit Einführung in Biopharmazie und Biotechnologie: BiblioScout; 2022. Available from: https://biblioscout.net/book/https://doi.org/10.52778/9783804743748.
Leuenberger H, Leu R. Formation of a tablet: a site and bond percolation phenomenon. J Pharm Sci. 1992;81(10):976–82.
York P. Crystal engineering and particle design for the powder compaction process. Drug Dev Ind Pharm. 1992;18(6–7):677–721.
Sun C, Grant DJ. Influence of crystal shape on the tableting performance of L-lysine monohydrochloride dihydrate. J Pharm Sci. 2001;90(5):569–79.
Huang Y, Khanvilkar KH, Moore AD, Hilliard-Lott M. Effects of manufacturing process variables on in vitro dissolution characteristics of extended-release tablets formulated with hydroxypropyl methylcellulose. Drug Dev Ind Pharm. 2003;29(1):79–88.
Levina M, Rajabi-Siahboomi AR. The influence of excipients on drug release from hydroxypropyl methylcellulose matrices. J Pharm Sci. 2004;93(11):2746–54.
Nokhodchi A, Ford JL, Rowe PH, Rubinstein MH. The influence of moisture content on the consolidation properties of hydroxypropylmethylcellulose K4M (HPMC 2208). J Pharm Pharm. 1996;48(11):1116–21.
Kottke MK, Rudnic EM. Tablet dosage forms. Modern Pharm. 2002;4:287–330.
Li Q, Rudolph V, Weigl B, Earl A. Interparticle van der Waals force in powder flowability and compactibility. Int J Pharm. 2004;280(1–2):77–93.
David S, Augsburger L. Plastic flow during compression of directly compressible fillers and its effect on tablet strength. J Pharm Sci. 1977;66(2):155–9.
Hiestand E, Wells J, Peot C, Ochs J. Physical processes of tableting. J Pharm Sci. 1977;66(4):510–9.
Roopwani R, Buckner IS. Understanding deformation mechanisms during powder compaction using principal component analysis of compression data. Int J Pharm. 2011;418(2):227–34.
Osei-Yeboah F, Chang S-Y, Sun CC. A critical examination of the phenomenon of bonding area-bonding strength interplay in powder tableting. Pharm Res. 2016;33:1126–32.
Ashby M. Mechanisms of deformation and fracture. Adv Appl Mechan. 1983;23:117–77.
Ge D. Mechanical metallurgy1988.
Werkmeister S, Dawson AR, Wellner F. Permanent deformation behaviour of granular materials. Road mater Pav Des. 2005;6(1):31–51.
Jones T, Ho A, Barker J. The use of instrumentation in tablet research, development and production. Pharm Technol. 1985;9:42–7.
Vromans H, Lerk C. Densification properties and compactibility of mixtures of pharmaceutical excipients with and without magnesium stearate. Int J Pharm. 1988;46(3):183–92.
Jetzer W. Compaction characteristics of binary mixtures. Int J Pharm. 1986;31(3):201–7.
Al-Ibraheemi ZAM, Anuar M, Taip F, Amin M, Tahir S, Mahdi AB. Deformation and mechanical characteristics of compacted binary mixtures of plastic (microcrystalline cellulose), elastic (sodium starch glycolate), and brittle (lactose monohydrate) pharmaceutical excipients. Partic Sci Technol. 2013;31(6):561–7.
Gentis ND, Betz G. Compressibility of binary powder formulations: Investigation and evaluation with compaction equations. J Pharm Sci. 2012;101(2):777–93.
Es-Saheb MH. The compaction characteristics of binary mixture powders. J King Saud Univ-Eng Sci. 1992;4(1):79–93.
Mattsson S, Nyström C. Evaluation of critical binder properties affecting the compactibility of binary mixtures. Drug Dev Ind Pharm. 2001;27(3):181–94.
Duberg M, Nyström C. Studies on direct compression of tablets XVII. Porosity—pressure curves for the characterization of volume reduction mechanisms in powder compression. Powder Technol. 1986;46(1):67–75.
Wu C-Y, Best SM, Bentham AC, Hancock BC, Bonfield W. Predicting the tensile strength of compacted multi-component mixtures of pharmaceutical powders. Pharm Res. 2006;23:1898–905.
Narayan BK, Hall K. Polyviriyl acetate applied to controlled-release formulations. Pharm Technol. 2003;18:34–7.
Kim Y-H, Om C-Y, Hwang Y-S, Hong Y-B. Adhesive properties of water-soluble and biodegradable hot-melt adhesive based on partially saponified poly (vinyl acetate). Mater Res Express. 2020;7(7):075301.
Basf A. Technical information for Kollidon SR. Germany: Ludwigshafen; 1999.
Picker K. The relevance of glass transition temperature for the process of tablet formation. J Ther Anal Calorimet. 2003;73:597–605.
Hossain MSIMS. Preparation and characterization of Ibuprofen matrix tablet based on Kollidon SR and Carnauba wax.USTA. 2007;13(1):86-92.
Draganoiu E, Andheria M, Sakr A. Evaluation of the new polyvinylacetate/povidone excipient for matrix sustained release dosage forms. Pharmazeutische Industrie. 2001;63(6):624–9.
Sahoo J, Murthy P, Biswal S, Sahoo S, Mahapatra A. Comparative study of propranolol hydrochloride release from matrix tablets with Kollidon® SR or hydroxy propyl methyl cellulose. Aaps Pharmscitech. 2008;9(2):577–82.
Saxena A, Srinivas N, Sravanthi M. Formulation and in–vitro evaluation of matrix type sustained release tablets of paliperidone. Innov Pharm Pharmacother. 2013;1(3):185–98.
Yasmin D, Rahman MR, Akter M. Formulation development of directly compressed Naproxen SR tablet using Kollidon SR and Avicel PH 102 polymer. Int CurrPharm J. 2013;2(6):112–4.
Kranz H, Guthmann C, Wagner T, Lipp R, Reinhard J. Development of a single unit extended release formulation for ZK 811 752, a weakly basic drug. Eur J pharm Sci. 2005;26(1):47–53.
Mohammad Safiqul Islam MSRMHR. In vitro release kinetics study of diltiazem hydrochloride from wax and kollidon SR based matrix tablets. Ind J Pharm Educ Res. 2008;7(2):101–8.
Mohammad Safiqul Islam MMRM. Effect of physiochemical properties on the release profile of Diltiazem HCl and Naproxen from HPMC and Kollidon SR based matrix tablets. Ind J Pharm Educ Res. 2009;43(1):46–54.
Shao ZJ, Farooqi MI, Diaz S, Krishna AK, Muhammad NA. Effects of formulation variables and post-compression curing on drug release from a new sustained-release matrix material: polyvinylacetate-povidone. Pharm Devel Technol. 2001;6(2):247–54.
Razzak SMI, Khan F, Hossain M, Khan ZR, Azad MAK, Reza S. Effect of channeling agents on the release pattern of theophylline from kollidon SR based matrix tablets. Pakistan J Pharm Sci. 2009;22(3).
Fouad EA, Ibrahim MA, El-Badry M. Embedment of chlorpheniramine maleate in directly compressed matrix tablets of compritol and kollidone SR. Tropic J pharm Res. 2015;14(3):371–7.
Das U, Hossain MS. Effects of release modifier on Carvedilol release from Kollidon SR based matrix. Int Curr Pharm J. 2012;1(8):186–92.
Sakr W, Alanazi F, Sakr A. Effect of Kollidon® SR on the release of Albuterol Sulphate from matrix tablets. Saudi Pharmaceut J. 2011;19(1):19–27.
Sarwar S, Hossain MS. Development and evaluation of sustained release losartan potassium matrix tablet using kollidon SR as release retardant. Brazilian J Pharmaceut Sci. 2012;48:621–8.
Hossain MA, Alam S, Paul P. Development and evaluation of sustained release matrix tablets of indapamide using Methocel K15M CR. J Appl Pharmaceut Sci. 2013;3(5):085–90.
Pragathi N. Effect of different diluents on release profile of Lamivudine from sustained release matrix tablet using kollidon SR as release retardant. Int J Res Pharmaceut Nano Sci. 2015;4(2):50–9.
Mulani H, Patel B, Shah N. Formulation and evaluation of Kollidon® SR for PH-independent extended release matrix systems for propranolol hydrochloride. J Pharm Sci Res. 2012;3.
Rahman MZ, Ahamed SK, Banik S, Hossain MS. Release profile of losartan potassium from formulated sustained release matrix tablet. Bangladesh Pharmaceut J. 2015;16(2):177–83.
Reza MS, Quadir MA, Haider SS. Development of theophylline sustained release dosage form based on kollidon SR. Pak J Pharm Sci. 2002;15(1):63–70.
Md. Selim Reza MAQSSH. Comparative evaluation of plastic, hydrophobic and hydrophilic polymers as matrices for controlled-release drug delivery. Pharm Pharm Sci. 2003;6(2):274-91.
T. Steenpaß, W. Fraunhofer, K. Kolter. The development of sustained release floating systems based on Kollidon® SR. Proc 3rd World Meeting on Pharm Biopharm and Pharm Technol. 2000;3:27-8.
Guguloth M, Bomma R, Veerabrahma K. Development of sustained release floating drug delivery. PDA J Pharm Sci Technol. 2011;65:198–206.
Shao ZJ, Farooqi MI, Diaz S, Krishna AK, Muhammad NA. Effects of formulation variables and post-compression curing on drug release from a new sustained-release matrix material: polyvinylacetate-povidone. Pharm Devel Technol. 2001;6(2):247–54.
Strübing S, Metz H, Mäder K. Characterization of poly (vinyl acetate) based floating matrix tablets. J Control Rel. 2008;126(2):149–55.
Mohan G, Ramesh B, Kishan V. Development of sustained release floating drug delivery system for norfloxacin. 2011;65(3):198–206.
Hauschild K, Picker-Freyer KM. Evaluation of tableting and tablet properties of Kollidon SR: the influence of moisture and mixtures with theophylline monohydrate. Pharm Devel Technol. 2006;11(1):125–40.
Engineer S, Shao ZJ, Khagani NA. Temperature/humidity sensitivity of sustained-release formulations containing Kollidon® SR. Drug Devel Ind Pharm. 2004;30(10):1089–94.
Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy: Lea & Febiger Philadelphia; 1976.
Denny P. Compaction equations: a comparison of the Heckel and Kawakita equations. Powder Technol. 2002;127(2):162–72.
Costa P, Lobo JS. Influence of dissolution medium agitation on release profiles of sustained-release tablets. Drug Devel Ind Pharm. 2001;27(8):811–7.
Yuksel N, Kanık AE, Baykara T. Comparison of in vitro dissolution profiles by ANOVA-based, model-dependent and-independent methods. Int J Pharm. 2000;209(1–2):57–67.
Picker KM, Hoag SW. Characterization of the thermal properties of microcrystalline cellulose by modulated temperature differential scanning calorimetry. J Pharm Sci. 2002;91(2):342–9.
Sun CC. Mechanism of moisture induced variations in true density and compaction properties of microcrystalline cellulose. Int J Pharm. 2008;346(1–2):93–101.
Roberts R, Rowe R. Brittle/ductile behaviour in pharmaceutical materials used in tabletting. Int J Pharm. 1987;36(2–3):205–9.
Rubinstein M. Tablets. Pharmaceutics: The science of dosage form design churchill livingstone. 1988:304-21.
Pesonen T, Paronen P. The effect of partice and power proterties on the mechnical properties of directly compressed cellulose tablets. Drug Devel Ind Pharm. 1990;16(1):31–54.
Westermarck S, Juppo AM, Kervinen L, Yliruusi J. Microcrystalline cellulose and its microstructure in pharmaceutical processing. Eur J Pharm Biopharm. 1999;48(3):199–206.
Albers J, Knop K, Kleinebudde P. Brand-to-brand and batch-to-batch uniformity of microcrystalline cellulose in direct tableting with a pneumohydraulic tablet press. Pharmazeutische Ind. 2006;68(12):1420–8.
Kushner J IV. Utilizing quantitative certificate of analysis data to assess the amount of excipient lot-to-lot variability sampled during drug product development. Pharm Devel Technol. 2013;18(2):333–42.
Bandelin FJ. Compressed tablets by wet granulation. Pharm Dosage Tab. 1989;1:131–93.
Reier G. Fun facts about Avicel® microcrystalline cellulose also known as cellulose gel. 2013.
Ilkka J, Paronen P. Prediction of the compression behaviour of powder mixtures by the Heckel equation. Int J Pharm. 1993;94(1–3):181–7.
Duberg M, Nyström C. Studies on direct compression of tablets XII. The consolidation and bonding properties of some pharmaceutical compounds and their mixtures with Avicel 105. Int J Pharm Tech Prod Manuf. 1985;6(2):17–25.
Barra J, Falson-Rieg F, Doelker E. Influence of the organization of binary mixes on their compactibility. Pharm Res. 1999;16:1449–55.
Agnese T, Cech T, Wildschek F. Determining the minimum amount of Kollidon® SR required in a tabletting blend to obtain a matrix tablet.
Humbert-Droz P, Mordier D, Doelker E. Densification behaviour of powder mixtures. Acta Pharm Technol. 1983;29(2):69–73.
Garr J, Rubinstein M. The effect of rate of force application on the properties of microcrystalline cellulose and dibasic calcium phosphate mixtures. Int J Pharm. 1991;73(1):75–80.
Sheikh-Salem M, Fell J. Compaction characteristics of mixtures of materials with dissimilar compaction mechanisms. Int J Pharm Tech Prod Mfr. 1981;2(1):19–22.
POLY(VINYL ACETATE) | 9003-20-7 2023 [Available from: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB3700594.htm. Accessed Aug 2023.
Sallmann AR. The history of diclofenac. Am J Med. 1986;80(Supplement 42):29–33.
Itoh T, Shohji E, Adachi S. Some physical properties of anhydrous alpha lactose stable form anhydrous alpha beta lactose compound and anhydrous beta lactose in crystalline state. Jpn J Zootech Sci. 1987;58(8):681–6.
Itoh T, Shohji E, Adachi S. Some physical properties of anhydrous alpha-lactose stable form, anhydrous alpha, beta-lactose compound and anhydrous beta-lactose in crystalline state. Jpn J Zootech Sci (Japan). 1987;58(8):681–6.
A. Maschke UKKK. Effect of copovidone on the release profile of theophylline from Kolllidon SR matrices. Development Pharma Ingredients.
Leuenberger H, Rohera B, Haas C. Percolation theory—a novel approach to solid dosage form design. Int J Pharm. 1987;38(1–3):109–15.
Holman L. The compaction behaviour of particulate materials. An elucidation based on percolation theory. Powder Technol. 1991;66(3):265–80.
Leuenberger H. The compressibility and compactibility of powder systems. Int J Pharm. 1982;12(1):41–55.
Holman L, Leuenberger H. The effect of varying the composition of binary powder mixtures and compacts on their properties: a percolation phenomenon. Powder Technol. 1990;60(3):249–58.
Blattner D, Kolb M, Leuenberger H. Percolation theory and compactibility of binary powder systems. Pharm Res. 1990;7:113–7.
Van Veen B, van der Voort MK, Bolhuis G, Zuurman K, Frijlink H. Tensile strength of tablets containing two materials with a different compaction behaviour. Int J Pharm. 2000;203(1–2):71–9.
Leuenberger H, Ineichen L. Percolation theory and physics of compression. Eur J pharm Sci Biopharm. 1997;44(3):269–72.
Kuentz M, Leuenberger H. A new theoretical approach to tablet strength of a binary mixture consisting of a well and a poorly compactable substance. Eur J Pharm Biopharm. 2000;49(2):151–9.
Bhat G, Hosmani A, Ketkar A, Paradkar A. Percolation theory: applications in pharmacy. Ind J Pharm Sci. 2000;62(5):321–6.
Ramı́rez N, Melgoza LMA, Kuentz M, Sandoval H, Caraballo I. Comparison of different mathematical models for the tensile strength–relative density profiles of binary tablets. Eur J pharm Sci. 2004;22(1):19-23.
Busignies V, Leclerc B, Porion P, Evesque P, Couarraze G, Tchoreloff P. Investigation and modelling approach of the mechanical properties of compacts made with binary mixtures of pharmaceutical excipients. Eur J Pharm Biopharm. 2006;64(1):51–65.
Amin MC, Fell JT. Comparison studies on the percolation thresholds of binary mixture tablets containing excipients of plastic/brittle and plastic/plastic deformation properties. Drug Devel Ind Pharm. 2004;30(9):937–45.
Jain N, Singh VK, Chauhan S. A review on mechanical and water absorption properties of polyvinyl alcohol based composites/films. J Mech Behav Mater. 2017;26(5–6):213–22.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15(1):25–35.
Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33.
Funding
This study received funding (grant 184/2021) from the Jordan University of Science and Technology (JUST).
Author information
Authors and Affiliations
Contributions
Wasfy M. Obeidat: conceptualization, study design, data collection, analysis, and interpretation; writing the paper. Ishraq K. Lahlouh helped in performing most of the experimental work and plotting the produced data. Shadi F. Gharaibeh provided assistance with the original manuscript’s writing, data analysis, and interpretation.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to update authors affiliation.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Obeidat, W.M., Lahlouh, I.K. & Gharaibeh, S.F. Investigations on Compaction Behavior of Kollidon®SR-Based Multi-component Directly Compressed Tablets for Preparation of Controlled Release Diclofenac Sodium. AAPS PharmSciTech 24, 225 (2023). https://doi.org/10.1208/s12249-023-02685-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02685-7